Lamellar hydroxide composite material and preparation method thereof
A layered hydroxide, composite material technology, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of limited application scope, lack of guest molecule selectivity, etc., to achieve good adsorption capacity and selectivity, high stability The effect of high specificity and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention also provides a method for preparing the layered hydroxide composite material, including: A) mixing layered rare earth hydroxides, crown ether carboxylic acid derivatives, water and alkali metal hydroxides, and reacting to obtain layered hydroxide composite material.
[0033] All the raw materials of the present invention have no special limitation on their sources, they can be purchased on the market or made by themselves. The layered rare earth hydroxides and crown ether carboxylic acid derivatives are the same as those described above, and will not be repeated here.
[0034] The preparation methods of the layered rare earth hydroxides and crown ether carboxylic acid derivatives can be prepared by methods well known to those skilled in the art, and there is no special limitation.
[0035] The present invention mixes layered rare earth hydroxides, crown ether carboxylic acid derivatives and water under the action of alkali metal hydroxides, where...
Embodiment 1
[0046] 1.1 will 2g Gd 2 o 3 Dissolved in 20ml of 1:1 nitric acid, heated and evaporated, concentrated and crystallized to obtain Gd(NO 3 ) 3 ·6H 2 O.
[0047] 1.2 Add 0.451g (1mmol) of Gd(NO 3 ) 3 ·6H 2 O, 1.105g (13mmol) NaNO 3 Mix with 0.140g (1mmol) hexamethylenetetramine, add 80ml of exhausted water, pass nitrogen gas for 5min, heat to 90°C for hydrothermal reaction for 12h, filter with suction, wash with water for 3 times to obtain white powder layered gadolinium Hydroxide is NO 3 - -LGdH.
[0048] 1.3 After dissolving 16.0g NaOH with 200ml water, put it in a 500ml three-necked round-bottomed flask, add 13.5ml ethylenediamine, control the temperature of the water bath at 40°C-50°C, and add 80.0g p-toluenesulfonyl chloride in batches while stirring ( TsCl), after continuous stirring for 4 hours, add 50ml of ethanol, heat up to 90°C, stop the reaction after reflux for 5min, cool to room temperature, filter with suction, wash the product three times with distilled...
Embodiment 2
[0061] Mix 0.075g (0.11mmol) of 4,7,13,16-tetracarboxyethyl-1,10-dioxo-4,7,13,16-tetraazacycloctadecane obtained in 1.6, 80ml of distilled water with Mix 0.77mmol sodium hydroxide, then add 0.10g of NO obtained in 1.2 3 - -LGdH, the pH value of the solution is about 9.38, stirred at room temperature for 24 hours, filtered by suction, and dried in vacuum to obtain a layered hydroxide composite material.
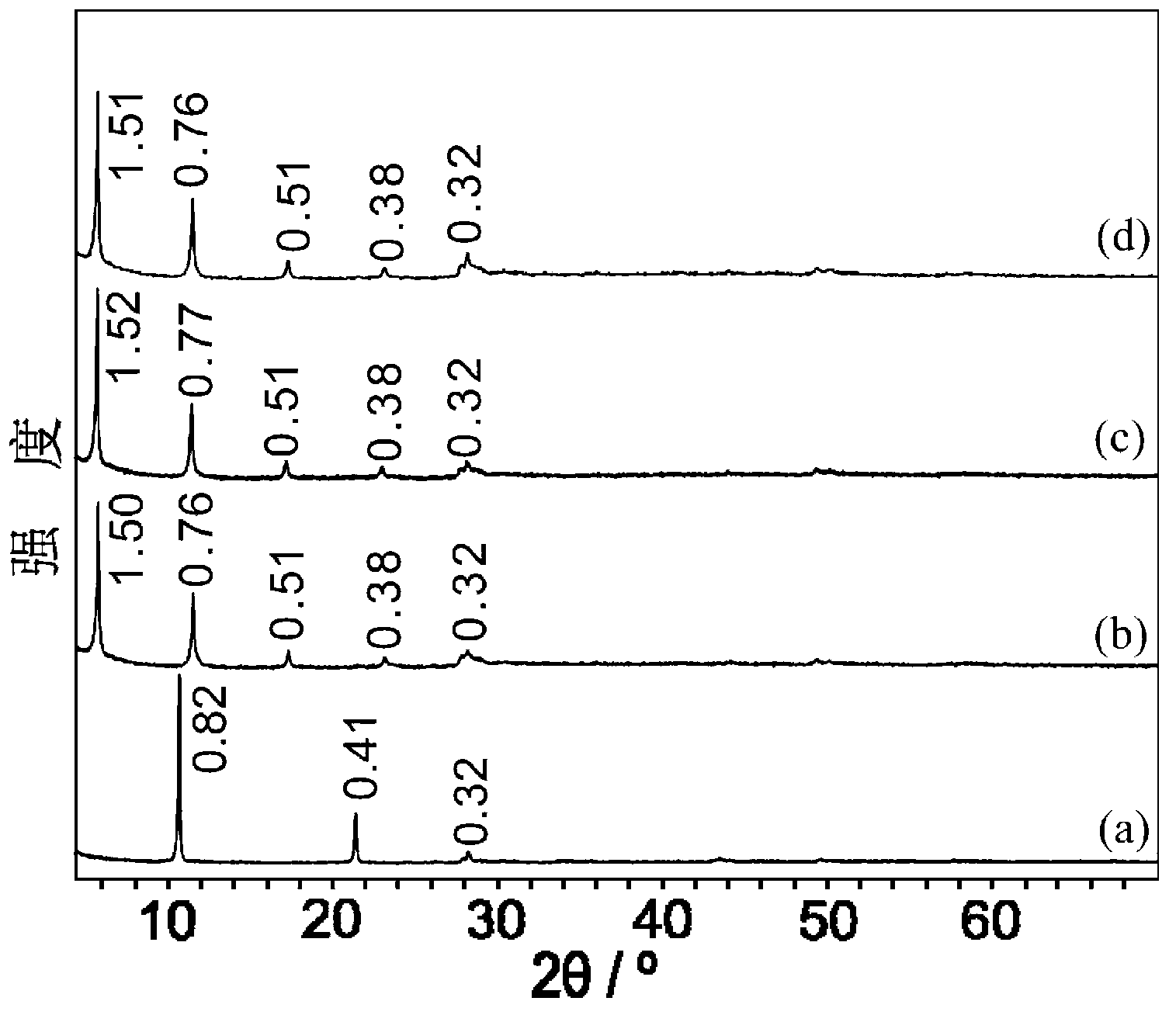
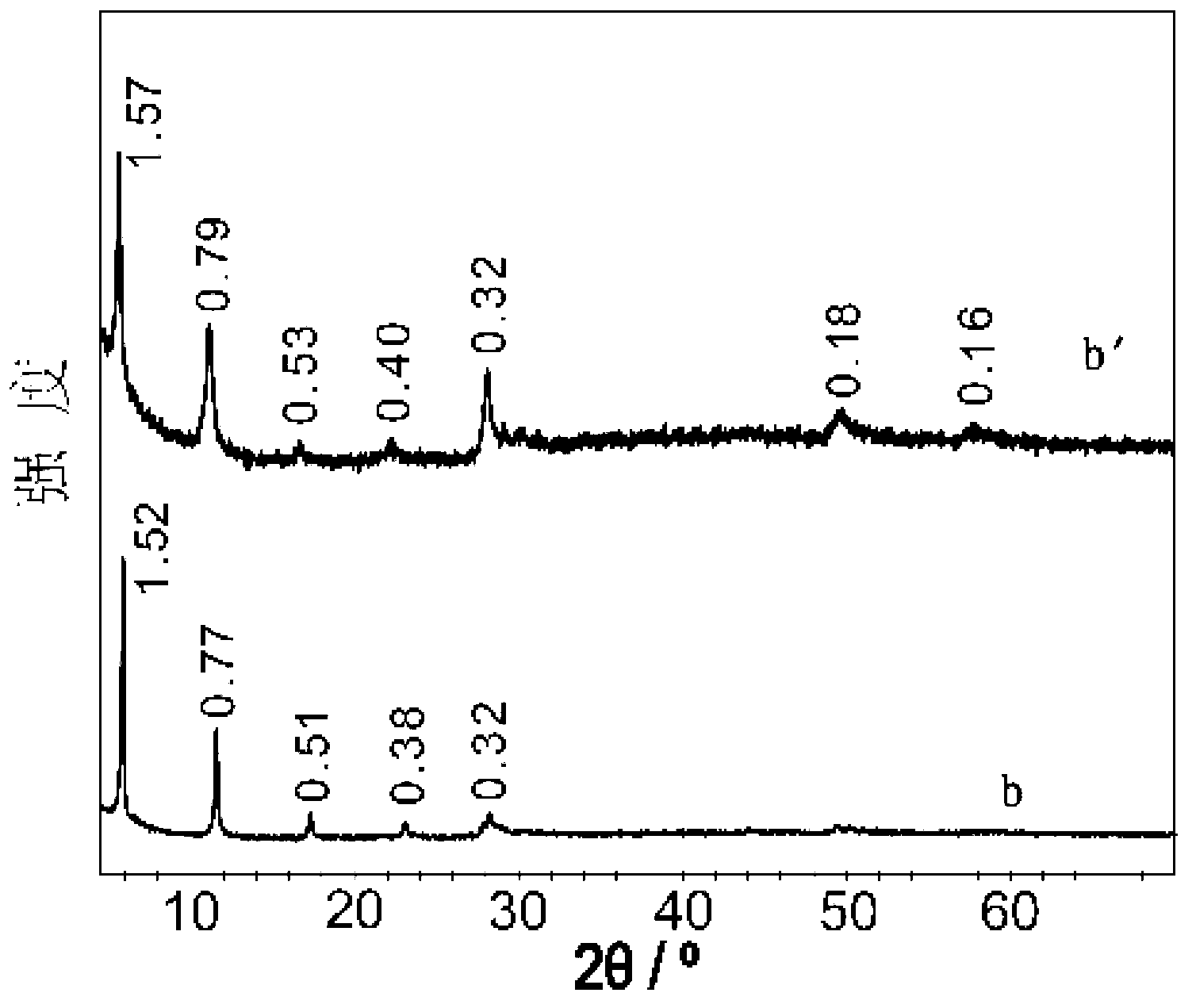
[0062] Utilize X-ray diffractometer to analyze the layered hydroxide composite material obtained in embodiment 2, obtain its X-ray diffraction pattern as figure 1 Shown, wherein c is the layered hydroxide composite material obtained in Example 2.
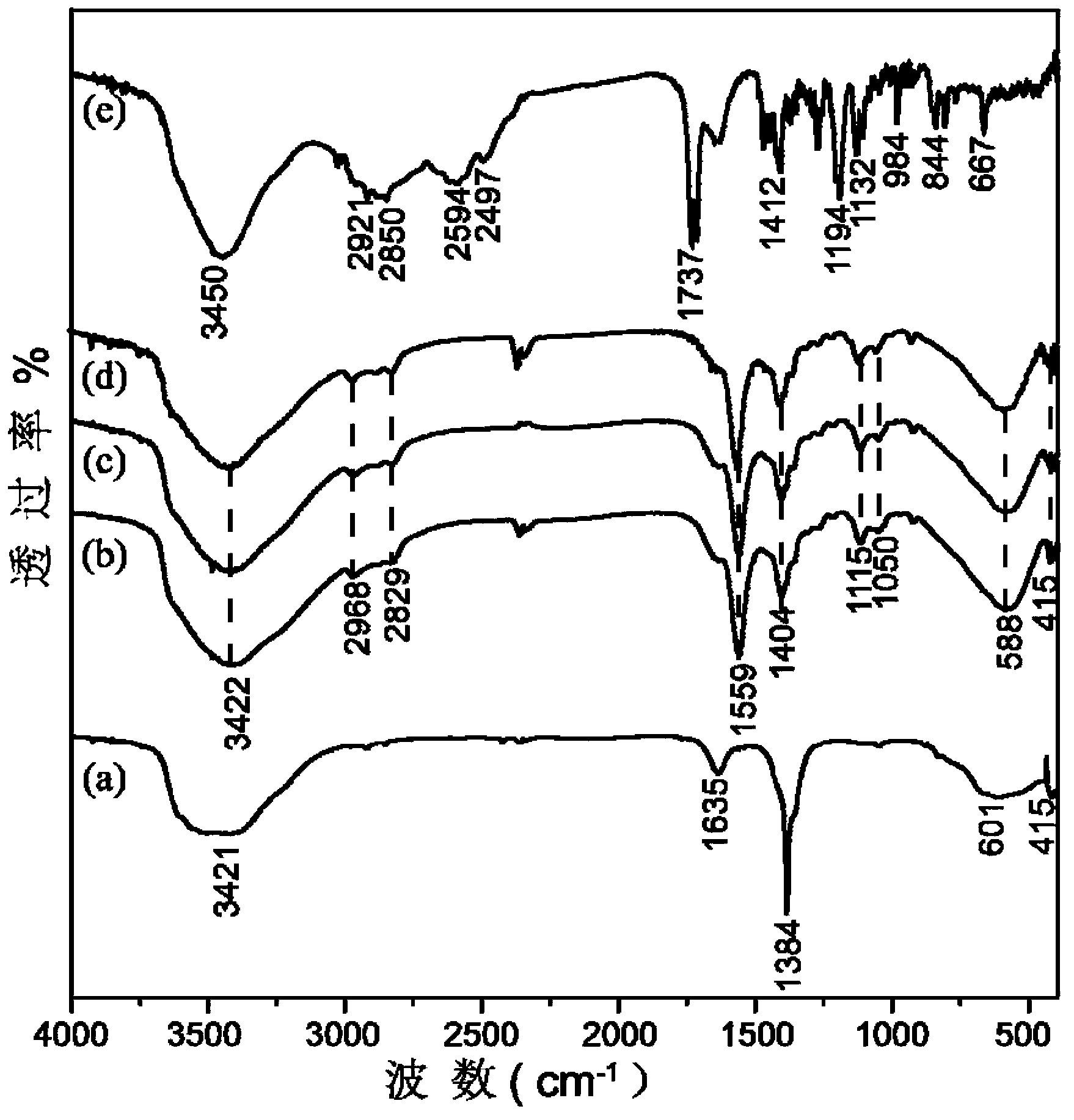
[0063] Utilize infrared spectrometer to analyze the layered hydroxide composite material obtained in embodiment 2, obtain its infrared spectrogram, as figure 2 shown. where c is the layered hydroxide composite obtained in 1.7.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com