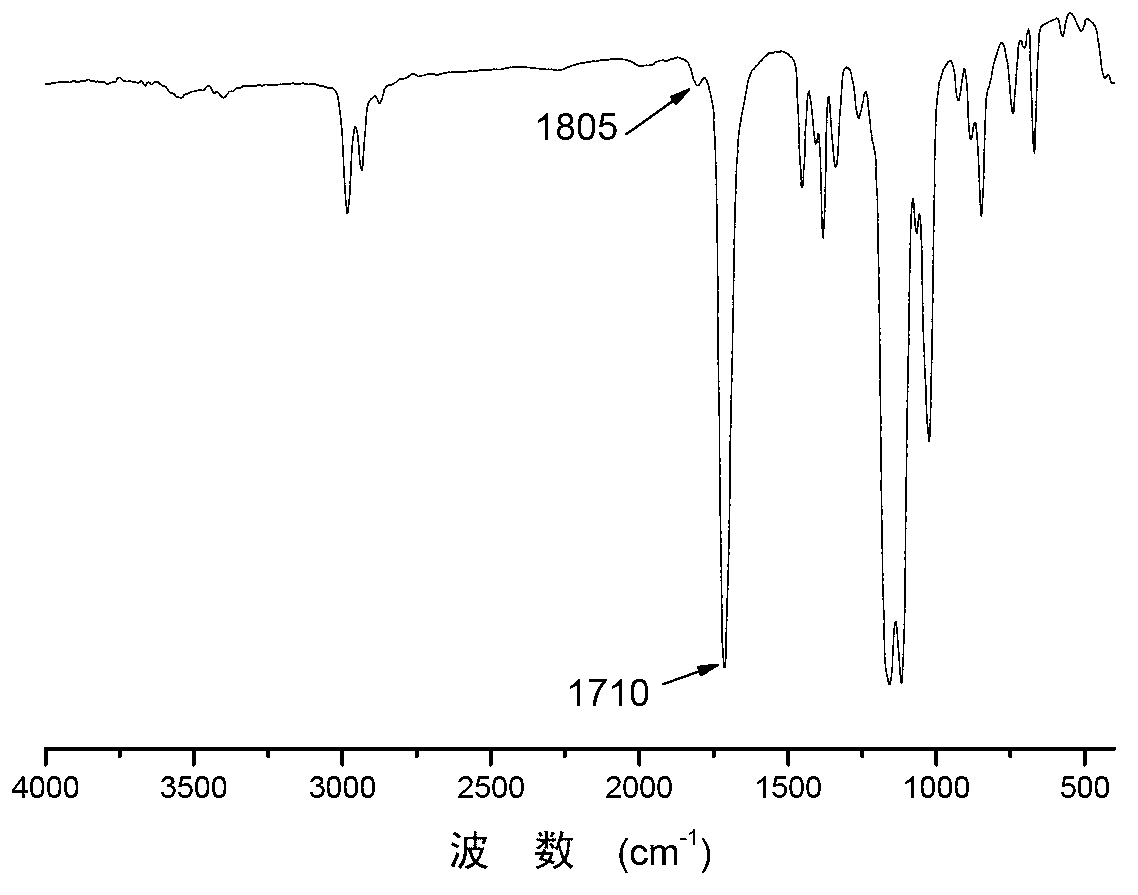
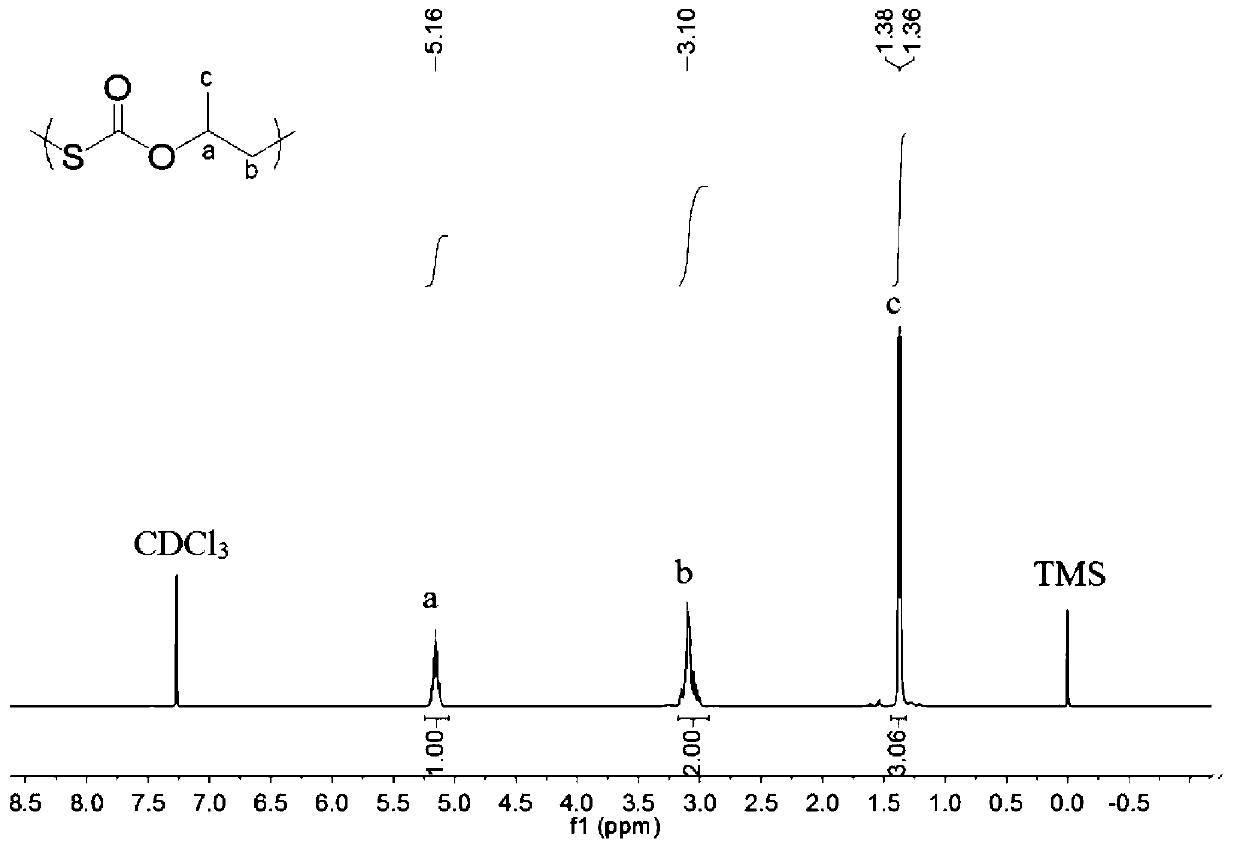
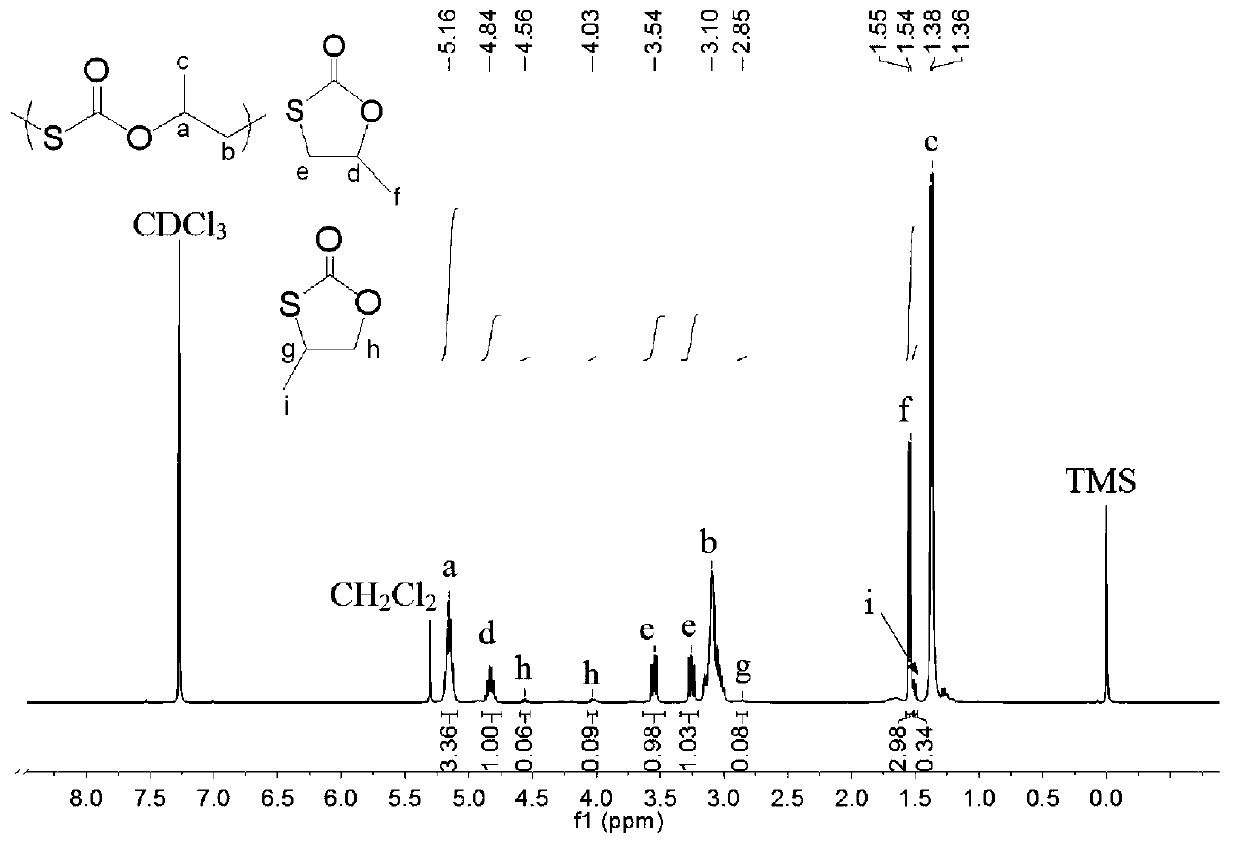
Preparation method of polymonosulfo-carbonate with regular chain structure
A technology of polymonothiocarbonate and chain structure, which is applied in the field of preparation of polymonothiocarbonate, can solve the problems of inability to effectively inhibit the oxygen-sulfur exchange reaction and lack of catalyst, and achieve easy operation, simple polymerization method, The effect of high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Before the polymerization reaction, the 10mL autoclave was dehydrated at 110°C for about 2 hours and cooled to room temperature in a desiccator. Add some quality SalenCrX catalyst 1a (such as figure 1 ) and cocatalyst N-methylimidazole (N-MeIm), both in equimolar numbers. The total molar ratio of the catalyst to the epoxy monomer is 1 / 10000. Then add 2.0 mL of 1,2-propylene oxide and the specified mass of COS (the molar ratio of COS to epoxy monomer is 0.5 / 1). Then the autoclave was closed and placed in an oil bath at 30 °C for 0.5 h under autogenous pressure. After the reaction was completed, it was cooled to room temperature, and the yellow product was taken out. The solvent was removed under reduced pressure, and then CH 2 Cl 2 The crude product was dissolved, the polymer was precipitated in methanol, and the yellow product was obtained after vacuum drying. The conversion rate was calculated by weighing method, and the content of each chain segment in the polyme...
Embodiment 2
[0045] Before the polymerization reaction, the 10mL autoclave was dehydrated at 110°C for about 2 hours and cooled to room temperature in a desiccator. Add some quality SalenCrX catalyst 2a (such as figure 1 ) and cocatalyst 4-dimethylaminopyridine (DMAP), both in equimolar numbers. The total molar ratio of the catalyst to the epoxy monomer is 1 / 10000. Then add 2.0 mL of 1,2-epoxybutane and the specified mass of COS (the molar ratio of COS to epoxy monomer is 0.5 / 1). Then the autoclave was closed and placed in an oil bath at 30 °C for 6 h under autogenous pressure. Other treatments are the same as in Example 1. The test results are shown in Table 1.
Embodiment 3
[0047] Before the polymerization reaction, the 10mL autoclave was dehydrated at 110°C for about 2 hours and cooled to room temperature in a desiccator. Add some quality SalenCrX catalysts 3a (such as figure 1 ) and cocatalyst bis(triphenylphosphoryl)ammonium chloride ([PPN]Cl), both in equimolar numbers. The total molar ratio of the catalyst to the epoxy monomer is 1 / 2000. Then add 2.0 mL of α-n-pentene oxide and the specified mass of COS (the molar ratio of COS to epoxy monomer is 1 / 1). Then the autoclave was closed and placed in an oil bath at 30 °C for 6 h under autogenous pressure. Other treatments are the same as in Example 1. The test results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com