Caspase 3-sensitive fluorescence enhancement nano developer and preparation method thereof
A caspase and fluorescence enhancement technology, applied in fluorescence/phosphorescence, material excitation analysis, peptide, etc., can solve problems such as poor stability, short excitation light wavelength, complex preparation, etc., and achieve good hydrophilicity and high sensitivity , good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
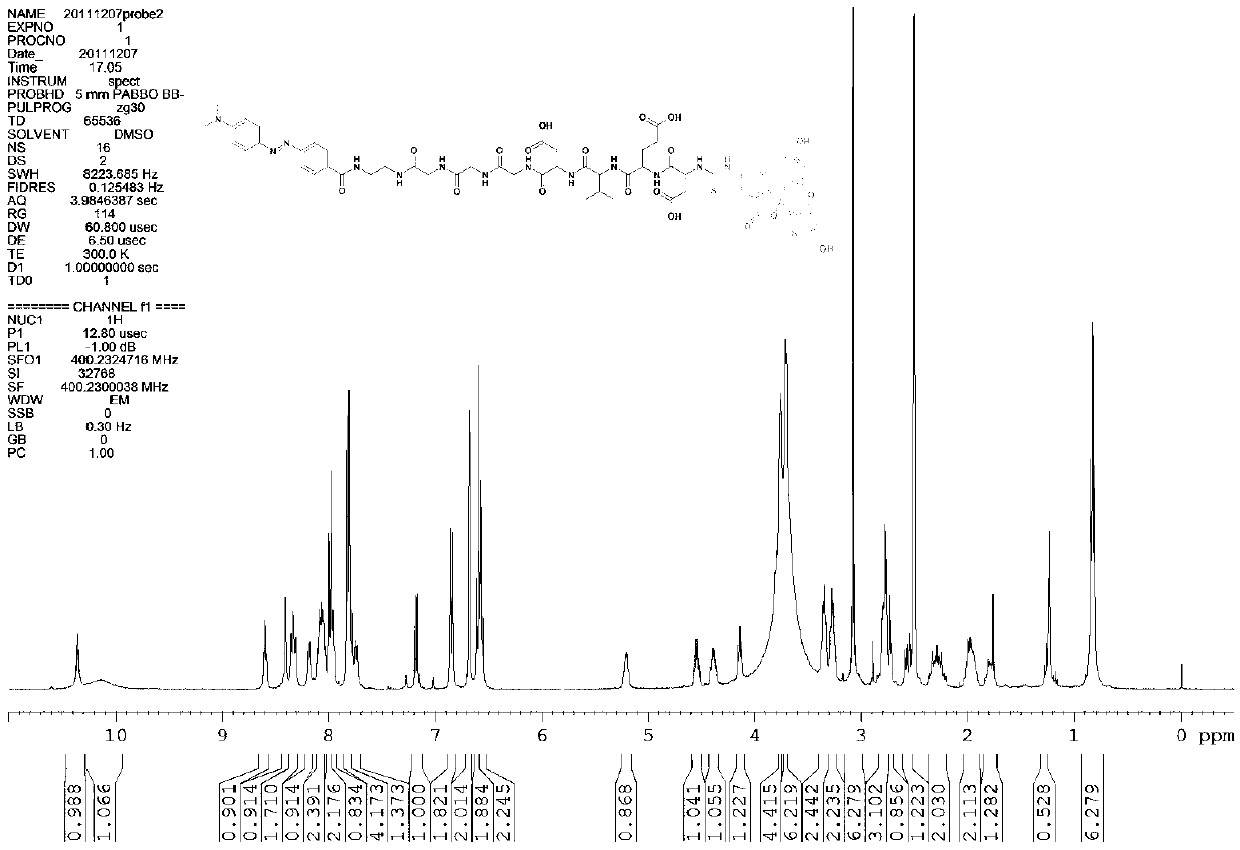
[0031] First, the oligopeptide fragment glycine-glycine-glycine-aspartic acid-valine-glutamic acid-aspartic acid-fluorenylmethoxycarbonyl is synthesized by solid-phase peptide synthesis.
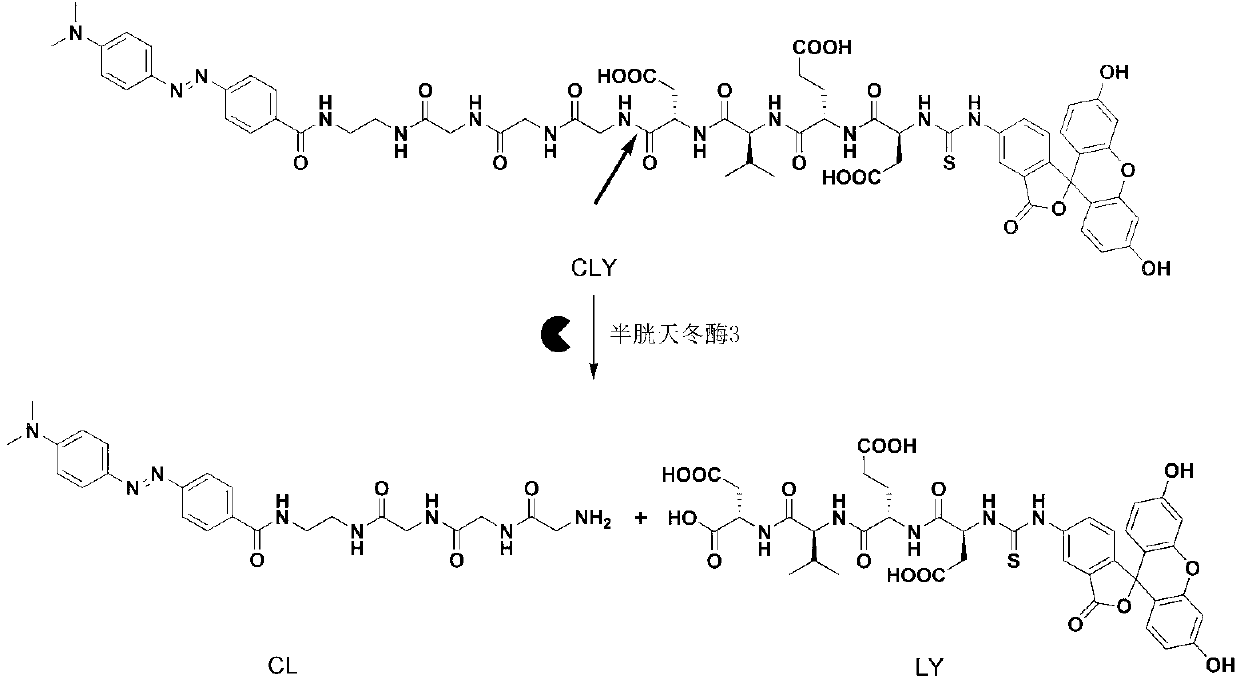
[0032] The synthesis route of the final compound CLY (Dabcyl-EDA-Gly-Gly-Gly-Gly-Asp-Val-Glu-Asp-FITC) in this example is as follows:
[0033]
[0034] .
[0035] First use the solid-phase peptide synthesis method to synthesize the oligopeptide sequence glycine-glycine-glycine-aspartic acid-valine-glutamic acid-aspartic acid-fluorenyl moxycarbonyl, press: 1 mmol 2-chlorotris Benzyl chloride resin was swollen in 2-3 ml of N, N-dimethylformamide for 4-8 minutes, and then 2 mmol of N-fluorenylmethoxycarbonyl-glycine was added, followed by 2 mmol of N, N-dimethylformamide. Isopropylethylamine, after reacting for 2-3 hours, react with 100 microliters of methanol for 5-10 minutes, add the mixed solution 4- 5 ml, react for 4-8 minutes, cut off the fluorenyl moxyprotecting group of glycine, Kai...
Embodiment 2
[0041] Embodiment 2: In vitro enzyme activity detection experiment
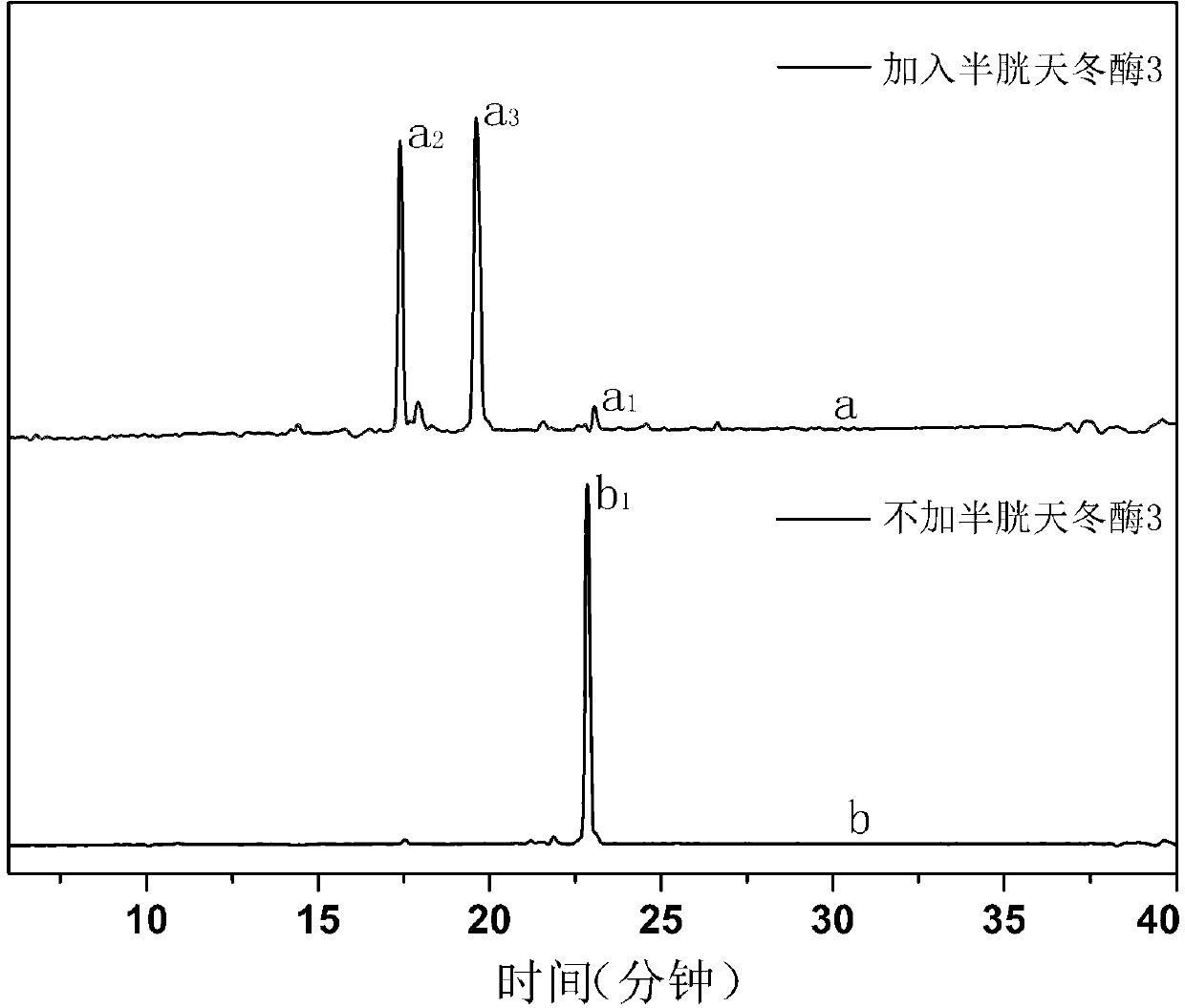
[0042] In the in vitro experiment of this embodiment, the final compound CLY with a concentration of 0.26 micromoles per liter was used, and the concentration was 50 millimoles per liter of 4-hydroxyethylpiperazineethanesulfonic acid, 0.1% of 3-[(3- cholesterylaminopropyl)dimethylamino]-1-propanesulfonic acid, 50 mmol per liter of sodium chloride, 10 mmol per liter of EDTA, and 5 cysteine with a unit volume of 50 microliters Aspartase 3 was incubated in a solution with a total volume of 100 microliters and incubated at 37°C for 80 minutes to complete the identification and cleavage of the enzyme. At the same time, the fluorescence emission spectrum was scanned to obtain the curve of the fluorescence emission spectrum changing with time. The detection of enzyme activity in the apoptotic cell lysate is carried out in the following way: 2 The human liver cancer cell line HepG2 was cultured in a 37°C incubator...
Embodiment 3
[0045] Example 3: Western blot experiment and apoptotic cell imaging experiment of UV-induced apoptotic cells
[0046] Firstly, the culture of human hepatoma cell HepG2 was carried out: at a volume concentration of 5% CO 2 The human liver cancer cell line HepG2 was cultured in a 37°C incubator in an air environment using DMEM medium containing 10% bovine serum albumin by volume; the cells in the logarithmic growth phase were washed three times with 0.01 moles per liter of sterile PBS buffer , and then digested with trypsin at a mass volume concentration of 0.25%; the sub-disc cells were rinsed with DMEM, then counted, and the cell concentration was diluted to 3×10 per ml 4 cells.
[0047] Then, test the changes of apoptosis-related proteases in human liver cancer cells HepG2 after ultraviolet induction and final compound CLY, and verify the influence of ultraviolet induction and final compound CLY on cells: human liver cancer cells HepG2 were divided into five groups, except ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com