Microencapsulated butylphthalide medicine composition, preparation method and applications
A technology of butylphthalide and composition, applied in the field of medicine, can solve problems such as research reports on the preparation method of butylphthalide pharmaceutical composition that have not yet been found, and achieve the effects of improving drug release behavior, high encapsulation rate and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
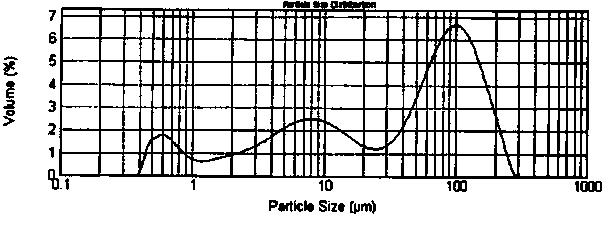
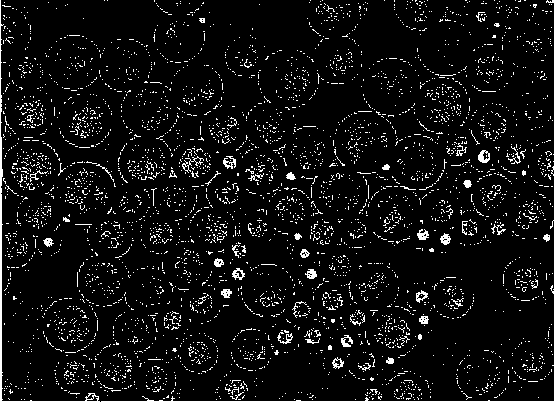
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of microencapsulated butylphthalide pharmaceutical composition by single coacervation method
[0048] Weigh 2.5 g of sodium alginate and 0.5 g of starch and dissolve them in distilled water so that the concentrations of sodium alginate and starch are 2.5% and 0.5% (w / w), respectively. Weigh 3.5g of racemic butylphthalide, dissolve it with a small amount of isooctane, add it to the sodium alginate-starch solution, disperse it evenly with a stirrer at 200r / min to make an emulsion, add 25% CaCl 2 Sodium alginate is made into calcium alginate to condense into capsules and solidified, filtered, washed with distilled water for 3-5 times, and vacuum-dried for 12 hours to obtain the microencapsulated butylphthalide pharmaceutical composition.
Embodiment 2
[0049] Example 2 Preparation of microencapsulated butylphthalide pharmaceutical composition by complex coacervation method
[0050] Weigh 3 g of gelatin, add 20 ml of water to fully swell, and keep warm to dissolve. Disperse 1.5g of L-butylphthalide in the gelatin solution and stir with a stirrer at 200 r / min for 30min until emulsified. Weigh 1.4g gum arabic, prepare a gum arabic solution with a concentration of 3.5%, add the levobutylphthalide-gelatin solution into the gum arabic in a linear form at a certain speed, adjust the pH to 4.0-4.5 with hydrochloric acid, and use an ice-water bath to Cool the solution to 5°C, cross-link and solidify the newly formed microcapsules with 25% glutaric acid amide, raise the solution system to room temperature, filter, wash off excess colloid and glutaric acid amide with distilled water, and vacuum dry for 12 hours to obtain Microencapsulated butylphthalide pharmaceutical composition.
Embodiment 3
[0051] Example 3 Preparation of microencapsulated butylphthalide pharmaceutical composition by spray drying method
[0052] Dissolve 10g of methylcellulose and 15g of sodium carboxymethylcellulose in distilled water to prepare spray solutions with a concentration of 10% and 15% respectively, and disperse 3g of L-butylphthalide in methylcellulose-carboxymethyl Sodium cellulose solution was stirred with a stirrer at 200 r / min for 30 minutes, and then spray-dried. The air inlet temperature is 135-145°C, the outlet temperature is 65-80°C, the feeding speed is 10 ml / min, and the compressed air flow rate is 600 NL / h, to obtain the microencapsulated butylphthalide pharmaceutical composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com