Method of low metal loading catalyst for preparing glycol from carbohydrate
A carbohydrate and catalyst technology, applied in the field of polyol preparation, can solve the problems of sintering and loss of catalyst active components, and achieve the effects of high dispersion, low cost and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Metal catalyst Ru / AC, Ru / SiO 2 ,Ru / ZnO,Ru / SBA-15, Ru / CaO,Ru / Al 2 O 3 ,Ru / TiO 2 Preparation: impregnate the activated carbon support with a mixed solution of 15wt% ruthenium trichloride aqueous solution and 20wt% sugar, dry at 120°C for 1 hour, then dry at 180°C for 6 hours, and finally roast in 350°C nitrogen atmosphere for 1 hour, respectively A Ru / AC catalyst with a loading amount of 0.05% Ru / C, 0.1% Ru / C, 0.2%, 0.5%, 0.7%, and 0.9% was obtained. The activated carbon carrier is replaced by alumina, silica, SBA-15, zinc oxide, titanium dioxide, calcium oxide, and the same method can be used to prepare catalysts supported by different carriers.
Embodiment 2
[0026] Catalytic conversion experiment: add 1.0g carbohydrates, 0.3g catalyst A, 0.03g catalyst B and 100ml water into a 300ml reactor, pass hydrogen to replace the gas three times, fill with hydrogen to 5MPa, heat up to 245°C and react for 30min. After the reaction, the temperature was lowered to room temperature, and the supernatant liquid after centrifugation was taken, separated on a calcium-type ion exchange column of high performance liquid chromatography and detected by a differential refractive index detector. In the product yield, only the target products ethylene glycol, propylene glycol, and hexahydric alcohols (including sorbitol, mannitol) are calculated. Other liquid products include butane erythritol, ethanol, unknown components, and gas products (CO 2 , CH 4 , C 2 H 6 Etc.) The yield was not calculated.
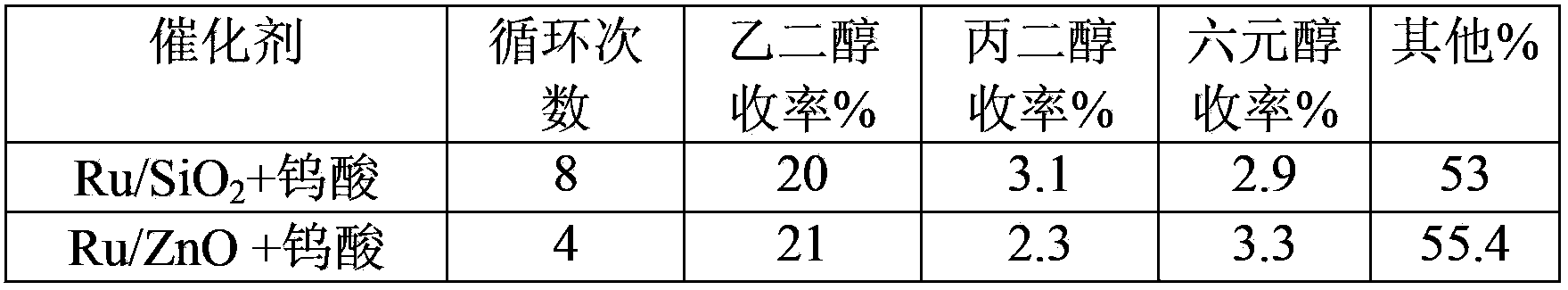
[0027] Ruthenium catalyst circulation reaction method: After the reaction, the ruthenium catalyst is separated by centrifugation and other methods, and used for t...
Embodiment 3
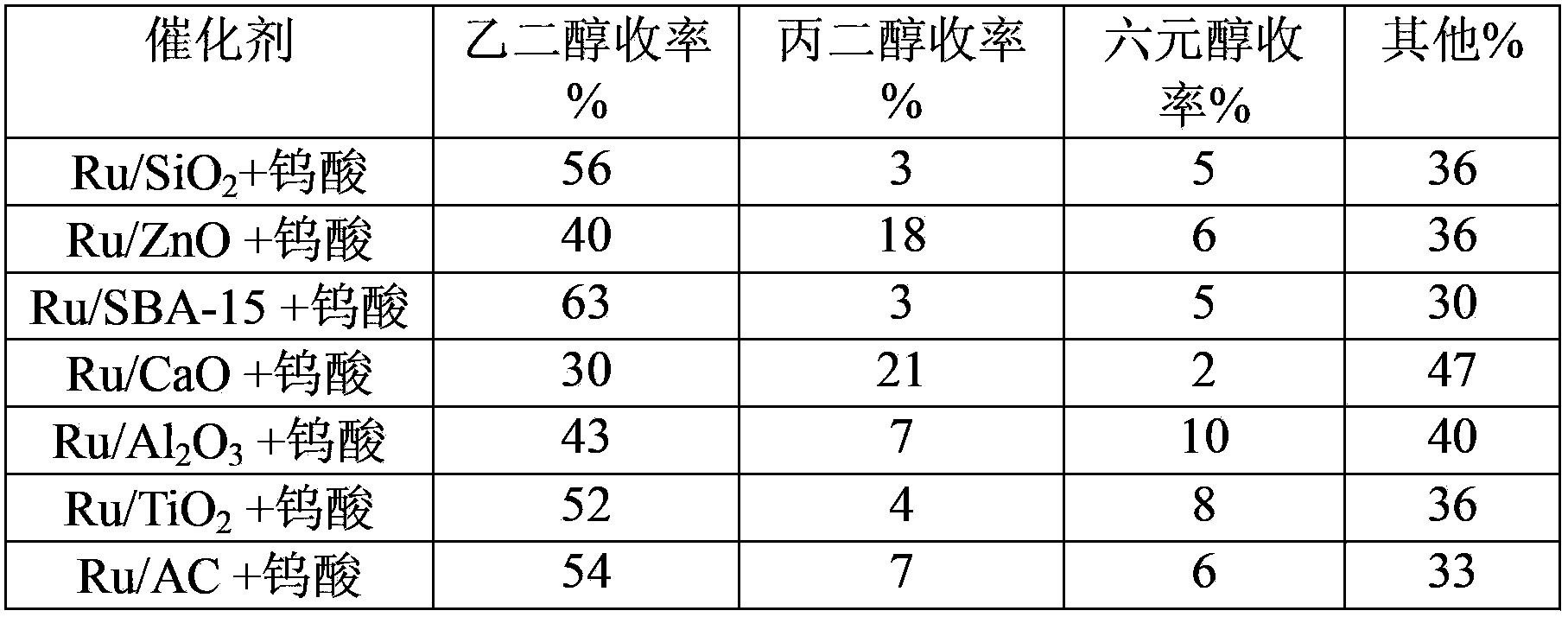
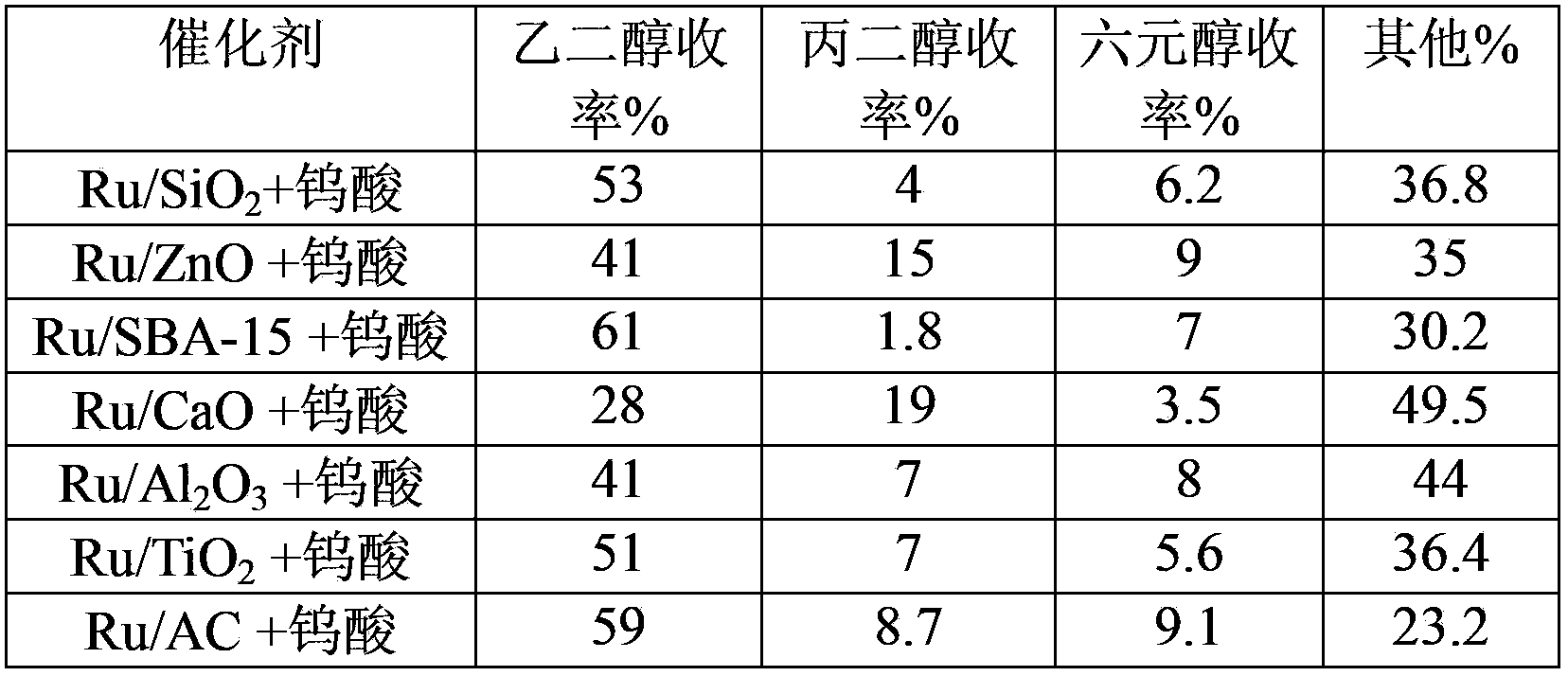
[0029] In the composite catalyst, the catalyst A was a Ru catalyst supported on different carriers, the loading amount was 0.9%, and the catalyst B was tungstic acid, and the reaction conditions were the same as in Example 2. Results of catalytic conversion of cellulose on various composite catalysts (Table 1 and Table 2).
[0030] Table 1 Results of catalytic conversion of cellulose during the first use of various catalysts
[0031]
[0032] Table 2 Results of catalytic conversion of cellulose after multiple cycles of various catalysts
[0033] Catalyst
[0034]
[0035] As shown in Table 1 and Table 2, cellulose can be converted into ethylene glycol with high yield on different composite catalysts in the catalytic process involved in the present invention and can be recycled for multiple times. Among them, the Ru / AC+tungstic acid combination can be recycled at least three times more than other catalyst combinations, reaching 45 times without significant reduction in activity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com