Medicinal sustained-release hydrogel as well as preparation method and application thereof
A hydrogel and drug technology, applied in the field of drug sustained-release hydrogel and its preparation, can solve the problems such as the research on the release rate and time of negative ions, the lack of application carrier in the drug sustained-release system, and the inability to achieve skin repair, so as to protect the drug. Bioactive, good elasticity, mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Experimental materials: sodium alginate (the viscosity of 2% aqueous solution at 25°C is 250cps), Sigma Company; polyvinyl alcohol (alcoholysis degree: 99.8~100%), Aladdin Company; carboxymethyl chitosan (deacetylation degree : ≥90, carboxylation degree: ≥80%), Guangzhou Feibo Biotechnology Co., Ltd.; heparin sodium (>170 units / mg), Bailingwei Company; bovine serum albumin (BSA), Genview Company; basic fibroblast growth Factor (bFGF), Biomedical Base of Jinan University.
[0037]High-voltage DC power supply (DW-P503-4ACCD), Tianjin Sanchuan Company; micro syringe pump (LSP01-1A), Baoding Lange Company; digital display air bath constant temperature oscillator (CHA-S), Jiangsu Jintan Co., Ltd.; syringe ( 2.5ml), Zhejiang Jinghuan Medical Supplies Co., Ltd.; polystyrene orifice plate (6, 24 holes), Nice Biotechnology Co., Ltd.
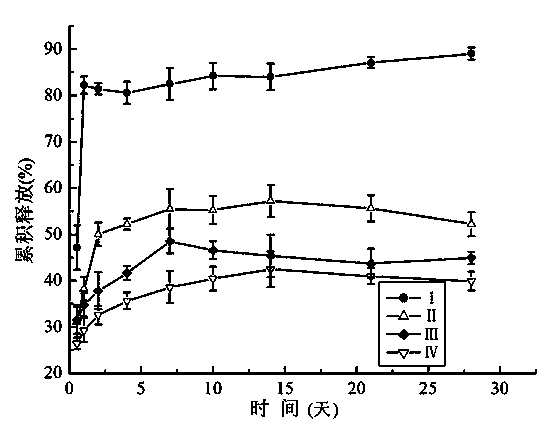
[0038] Experimental content: Calcium alginate microspheres were prepared by high-voltage electrostatic droplet method, with 10mg / ml BSA and 2wt% ...
Embodiment 2
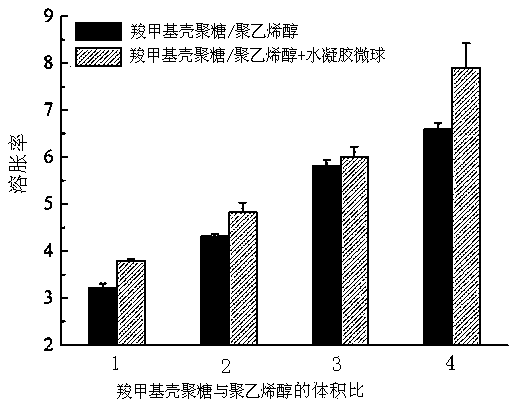
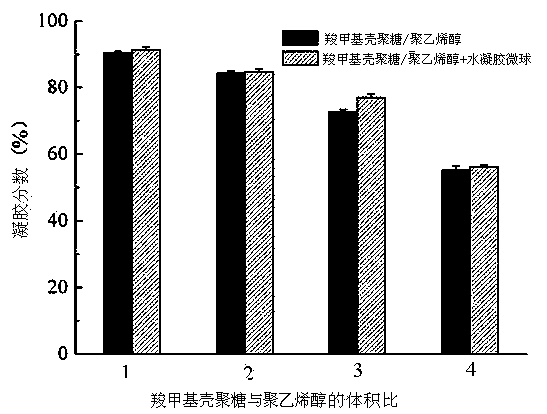
[0046] Calcium alginate microspheres were prepared by high-voltage electrostatic droplet method, using 1.17 μg / ml basic fibroblast growth factor (bFGF)-containing 1wt% heparin aqueous solution and 2wt% sodium alginate aqueous solution as raw materials, 3% (w / v) CaCl 2 The aqueous solution is used as the receiver, the inner diameter of the needle is 200μm, and the needle tip is kept in contact with CaCl 2 The liquid surface distance of the solution is 20 cm, and the electric field voltage is 10 kV. Cured for 10 minutes, washed three times with deionized water. Use filter paper to filter out the moisture on the surface of the microspheres, weigh 50 mg of calcium alginate microspheres and add them to two uniformly mixed hydrogel matrices, which contain 2% carboxymethyl chitosan and 10% polyvinyl alcohol solution, its volume is 2:4, vortex for a few seconds to make it evenly dispersed. The hydrogel containing calcium alginate microspheres was frozen at -20°C for 20 h, and then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com