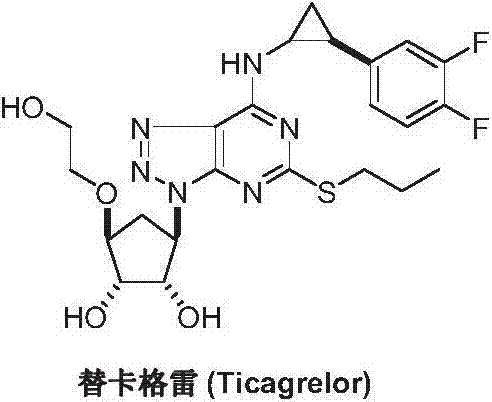
Method for preparing ticagrelor intermediate
A technology of ticagrelor and intermediates, which is applied in the design of organic synthesis routes and the preparation of raw materials and intermediates, can solve the problems of long steps, difficult separation, rare raw materials and the like, and achieves the reaction conditions suitable for large-scale industrial production. Easy to control, mild reaction conditions effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add (1R,2R,3R.4R)-rel-1,2;3,4-dioxycyclopentane (I) (9.8g, 0.1mol), sodium azide (8.13g, 0.125mol) and acetone / water (1 / 1) mixed solvent 200mL, start stirring, raise the temperature to 45-50°C, react for 72 hours, and the reaction is completed by GC detection. Diethyl ether was added until the solution was separated and the organic phase was separated. The aqueous phase was extracted 5 times with 100 mL of ether and acetone (4:1). The organic phases were combined and dried over anhydrous magnesium sulfate. Concentrate under reduced pressure, and the residue is subjected to column chromatography (ether / n-pentane=5 / 1) to obtain a white solid (3aR,4S,6R,6aS)-rel-6-azidotetrahydro-2,2-dimethyl 9.0 g of yl-4H-cyclopenta-1,3-dioxan-4-ol (II), yield 45.2%.
Embodiment 2
[0034]Under nitrogen protection, add (3aR,4S,6R,6aS)-rel-6-azidotetrahydro-2,2-dimethyl-4H-cyclopenta-1,3-diox Hetero-4-alcohol (II) (8.0g, 0.04mol), tetrabutylammonium bromide (phase transfer catalyst) 0.25g and dry toluene 100mL, add sodium hydride (1.92g, 0.08mol) in batches under stirring, drop Add 2-bromoethanol (9.92 g, 0.08 mol) and slowly warm to reflux. Keep the reflux reaction for about 24 hours, and TLC detects that the reaction is complete. Cool to room temperature, add water and stir for 15 minutes, separate layers. The organic phase was washed with brine and dried over anhydrous sodium sulfate. The solvent was recovered by distillation under reduced pressure, and the residual oil was recrystallized from n-hexane to obtain a white solid 2-[[(3aR,4S,6R,6aS)-rel-6-azidotetrahydro-2,2-dimethyl- 8.1 g of 4H-cyclopenta-1,3-dioxan-4-yl]oxy]ethanol (III), yield 83.3%.
Embodiment 3
[0036] Under nitrogen protection, add 2-[[(3aR,4S,6R,6aS)-rel-6-azidotetrahydro-2,2-dimethyl-4H-cyclopenta-1 into the reaction flask, 3-Dioxa-4-yl]oxy]-ethanol (III) (7.3g, 0.03mol), Hans ester 1,4-dihydropyridine (9.1g, 0.036mol), 10% palladium carbon 1.8g and ethanol 50mL, started stirring, heated to reflux and maintained reflux for 5 hours, and TLC detected that the reaction was complete. Cool to room temperature, filter, and recover the catalyst. Concentrate under reduced pressure, dissolve the residue in dichloromethane, and wash successively with 5% hydrochloric acid, saturated brine and water. Dry over anhydrous sodium sulfate, and recover the solvent by distillation under reduced pressure to obtain light yellow oil 2-[[(3aR,4S,6R,6aS)-rel-6-aminotetrahydro-2,2-dimethyl-4H-cyclo Penteno-1,3-dioxan-4-yl]oxy]ethanol (IV) 5.9 g, yield 90.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com