Aspirin enteric-coated tablet and preparation technology thereof
An aspirin and preparation process technology, applied in the field of pharmaceutical preparations, can solve the problems of lack of buffer and exhaust, irreversible damage of punching tools, high risk, etc., to enhance compressibility and fluidity, improve production safety factor, and solve solvent problems. residual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
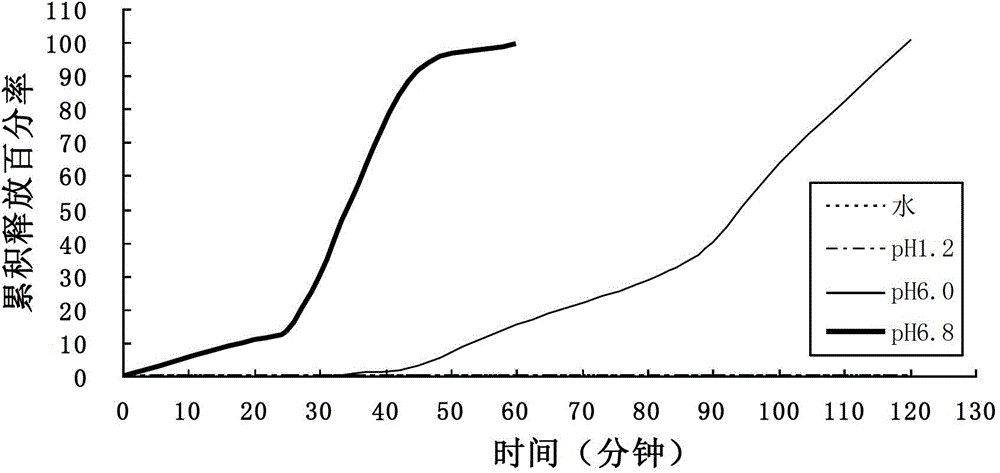
[0043] Calculated according to the weight of 1000 tablets, aspirin 100g, lactose 10.125g, microcrystalline cellulose 10.125g, 10% starch slurry 2.375g, talcum powder 7.5g; L30D-55 accounts for 8.75g, Ute NE30D accounted for 1.25g, triethyl citrate 1.25g, polysorbate-80 accounted for 0.625g, glyceryl monostearate 0.625g, and the solvent was water.
[0044] According to the above formula, (1) mix lactose and microcrystalline cellulose through a wet granulator, Ⅱ stirring Ⅰ shearing, a total of 2-5 minutes; (2) granulate with 10% starch slurry through a wet granulator, Ⅰ stirring Ⅱ Shearing, a total of 2-5 minutes, drying through a box-type drying oven or a fluidized granulation dryer at a drying temperature of 50-90°C; (3) Mixing blank granules and aspirin through a three-dimensional mixer or a column mixer, parameters: 500-800mA, 25-45 minutes; (4) Granulate the mixed granules through a dry granulator, parameters: pressure 3-6Kg, speed 500-900mA; (5) Mix and dry press throug...
Embodiment 2
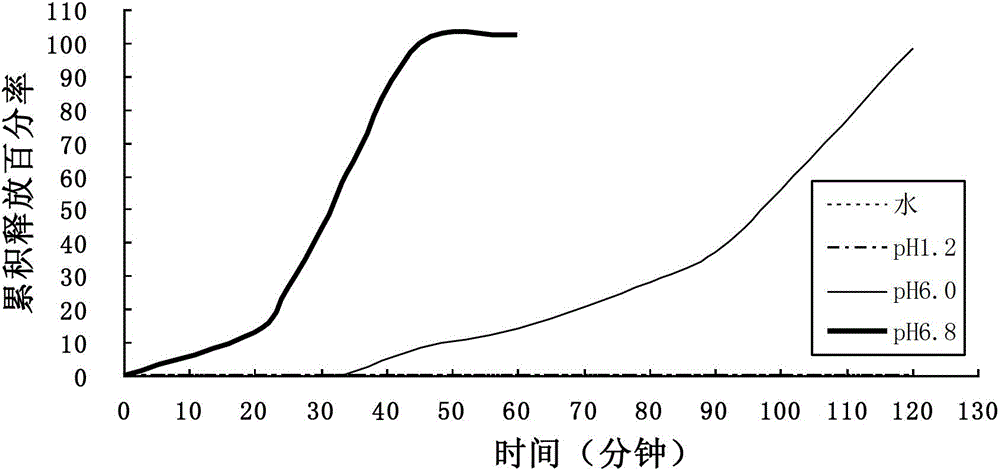
[0047] Based on the weight of 1000 tablets, aspirin 100g, starch 20g, 2% hypromellose appropriate amount, talcum powder 0.615g; the proportion of enteric-coated layer by weight of solids is: uterine L100-55 accounted for 9.84g, triethyl citrate 1.476g, polysorbate-80 accounted for 0.369g, glyceryl monostearate 0.615, and the solvent was water.
[0048] According to the above formula, the process of the present invention is used for tablet compression and coating, and the release statistics are shown in the table below.
[0049]
Embodiment 3
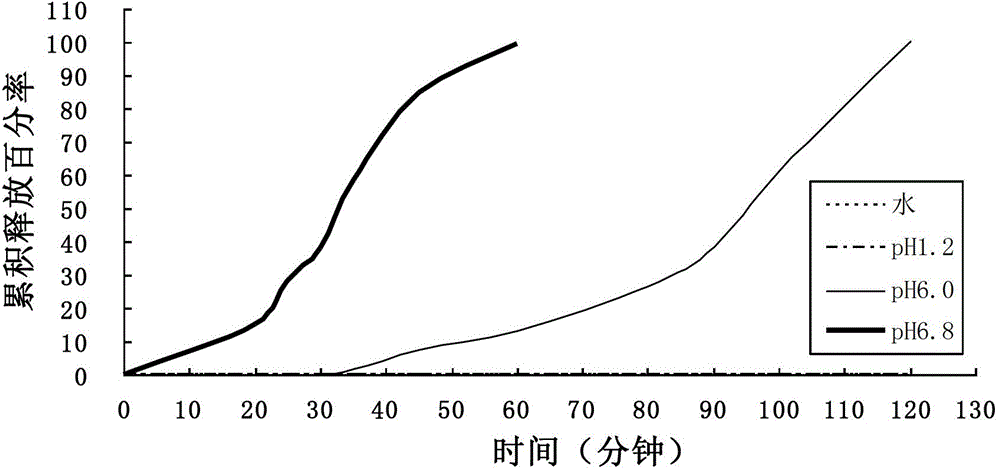
[0051] Based on the weight of 100 tablets, aspirin 100g, starch 11.08g, lactose 7.38, pregelatinized starch 5.54g, 2% hypromellose appropriate amount; the proportion of enteric-coated layer by weight of solids is: Ute L100-55 accounts for 4.92g, Ute NE30D accounted for 2.952g, triethyl citrate 0.492g, polysorbate-80 accounted for 0.1968g, glyceryl monostearate 0.2952g, talc powder 0.984g, and the solvent was water.
[0052] According to the above formula, the process of the present invention is used for tablet compression and coating, and the release statistics are shown in the table below.
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com