Rosinyl quaternary ammonium salt dimeric surfactant as well as preparation method and application thereof
A technology of rosin-based quaternary ammonium salts and gemini surfaces, which is applied in the preparation of amino compounds, botany equipment and methods, applications, etc., can solve the problems that the research on rosin-based heterogemini surfactants has not been reported, and achieve good surface activity and Bacteriostatic properties, high efficiency, and short-term effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
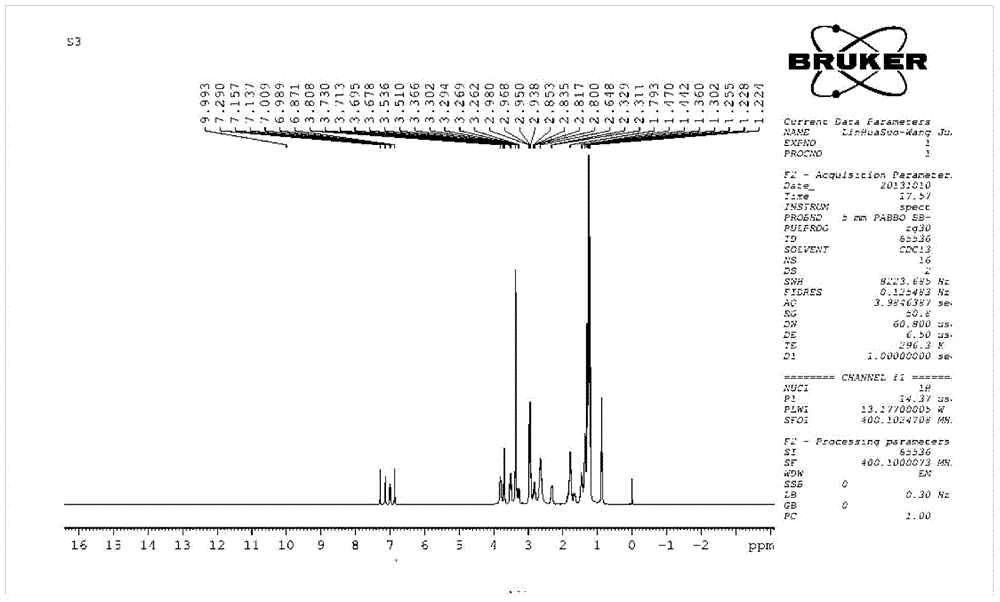
[0039] Synthesis of N,N-dimethylabietinamine:
[0040] With dichloromethane as solvent, SOCl 2 (2.4g, 20mmol) and abietic acid (3.0g, 10mmol) reacted 4h at reflux temperature, removed solvent and excess SOCl 2 Obtain abietic acid chloride. Dissolve the obtained abietic acid chloride in 20mL of tetrahydrofuran, slowly add it dropwise into 40% dimethylamine aqueous solution (4.5g, 40mmol) at 0°C and react for 4h. After the reaction is completed, filter, and the filtrate is acidified and alkalized. 1. After washing with water, distill off the solvent under reduced pressure to obtain N,N-dimethyl dehydroabietamide. Finally, N,N-dimethyl dehydroabietamide was dissolved in tetrahydrofuran solvent, lithium aluminum hydride (0.8g, 20mmol) was added with stirring at 0°C, and the temperature was slowly raised to 70°C and reacted for 6h. After the reaction is completed, continue to dropwise add water equivalent to the quality of lithium aluminum hydride and 15% sodium hydroxide soluti...
Embodiment 2
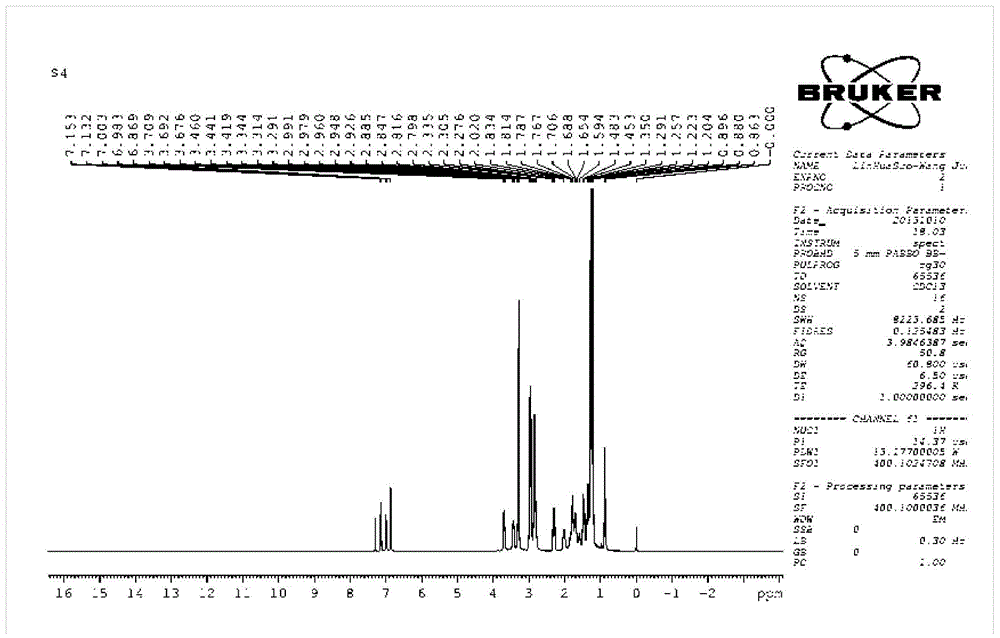
[0042] Synthesis of bromoalkyl abietyl dimethyl ammonium bromide:
[0043] (1) Synthesis of 3-bromopropyl abietyl dimethyl ammonium bromide (n=3):
[0044] Take N,N-dimethyl abietamine (3.1g, 10mmol) in a reactor filled with absolute ethanol, add 1,3-dibromopropane (2.0g, 10mmol) dropwise under stirring, and react at 50°C for 30h Afterwards, the temperature was raised to 80° C., and the reaction was continued for 8 hours. After the reaction was completed, the solvent was distilled off under reduced pressure, excess dibromopropane was washed off with petroleum ether, and dried to obtain 3-bromopropyl abietyl dimethyl ammonium bromide.
[0045] (2) Synthesis of 4-bromobutyl abietyl dimethyl ammonium bromide (n=4):
[0046] Take N,N-dimethyl abietamine (3.1g, 10mmol) in a reactor filled with absolute ethanol, add 1,4-dibromobutane (7.4g, 35mmol) dropwise under stirring, and react at 50°C After 24h, react at 80°C for another 24h. After the reaction was completed, the solvent w...
Embodiment 3
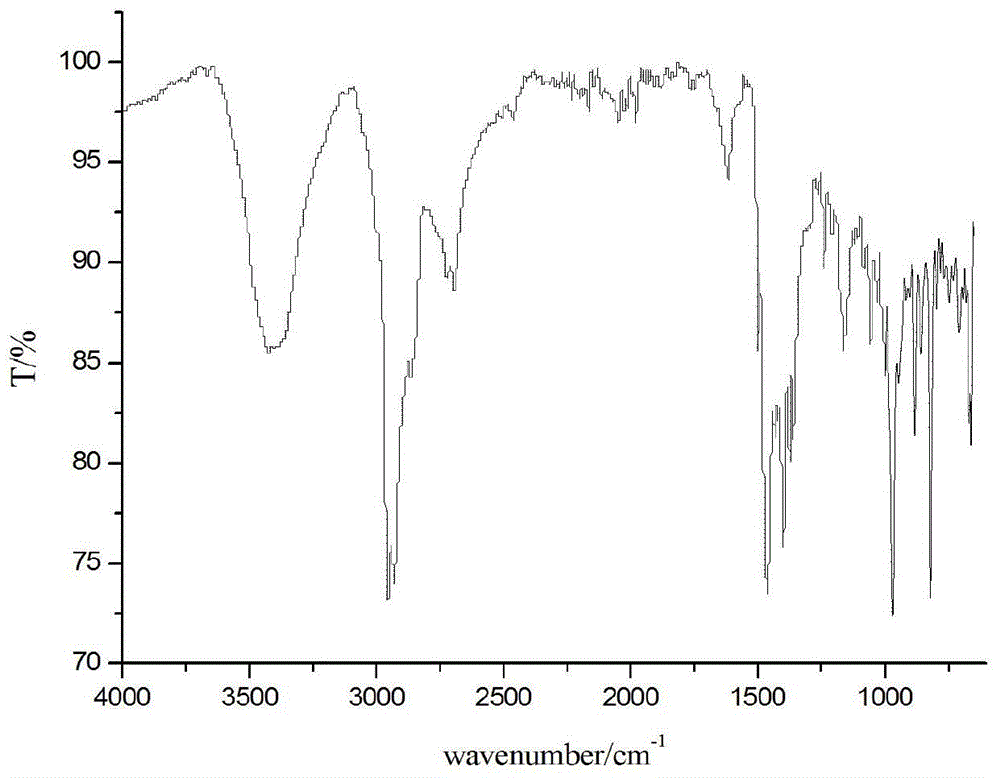
[0050] Synthesis of rosin-based quaternary ammonium salt heterogemini surfactant:
[0051] (1) Synthesis of conventional heating rosin-based quaternary ammonium salt heterogemini surfactant A:
[0052] Add N,N-dimethyldodecylamine (2.3g, 10.5mmol) and 3-bromopropyl abietyl dimethylammonium bromide ( 4.1g, 10mmol) and 100ml acetone, heated, stirred and refluxed for 36h. After the reaction was completed, the solvent was distilled off under reduced pressure, followed by recrystallization and vacuum drying to obtain the rosin-based quaternary ammonium salt heterogemini surfactant A with a yield of 70%.
[0053] (2) Synthesis of Rosin-based Quaternary Ammonium Heterogemini Surfactant A by Microwave Radiation:
[0054] Add N,N-dimethyldodecylamine (2.3g, 10.5mmol) and 3-bromopropyl abietyl dimethylammonium bromide (4.1g, 10mmol) to a 100ml round bottom single-necked bottle, in 40ml acetone Dissolved in a microwave reactor and placed in a microwave reactor, the microwave reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com