Synthesis and application of fluorescence molecular probe containing cyanogens ions by naked eyes and fluorescence ratio detection
A technology of fluorescent molecular probe and cyanide ion, which is applied in the field of chemical analysis and detection, can solve the problems of poor selectivity, expensive equipment, complicated processing, etc., and achieve the effects of low detection limit, wide detection range and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of diethylaminocoumarin aldehyde
[0026]Under the protection of argon, 2mL of N,N-dimethylformamide was cooled in an ice bath and vigorously stirred, slowly added dropwise with 2mL of phosphorus oxychloride, and continued to stir for 1 hour after the dropwise addition; then diethylcoumarin The element (1.33g.6.12mmol) was dissolved in 10mL of anhydrous N,N-dimethylformamide, added dropwise to the above solution, and the temperature was raised to 70°C for 18 hours. After the reaction was completed, it was lowered to room temperature, poured into 100 mL of ice water, stirred vigorously, and an orange-yellow solid was precipitated. Suction filtration, washing with water and ethanol, and recrystallization with absolute ethanol to obtain 0.6 g of yellow needle-like crystals, namely diethylaminocoumarin aldehyde (40%).
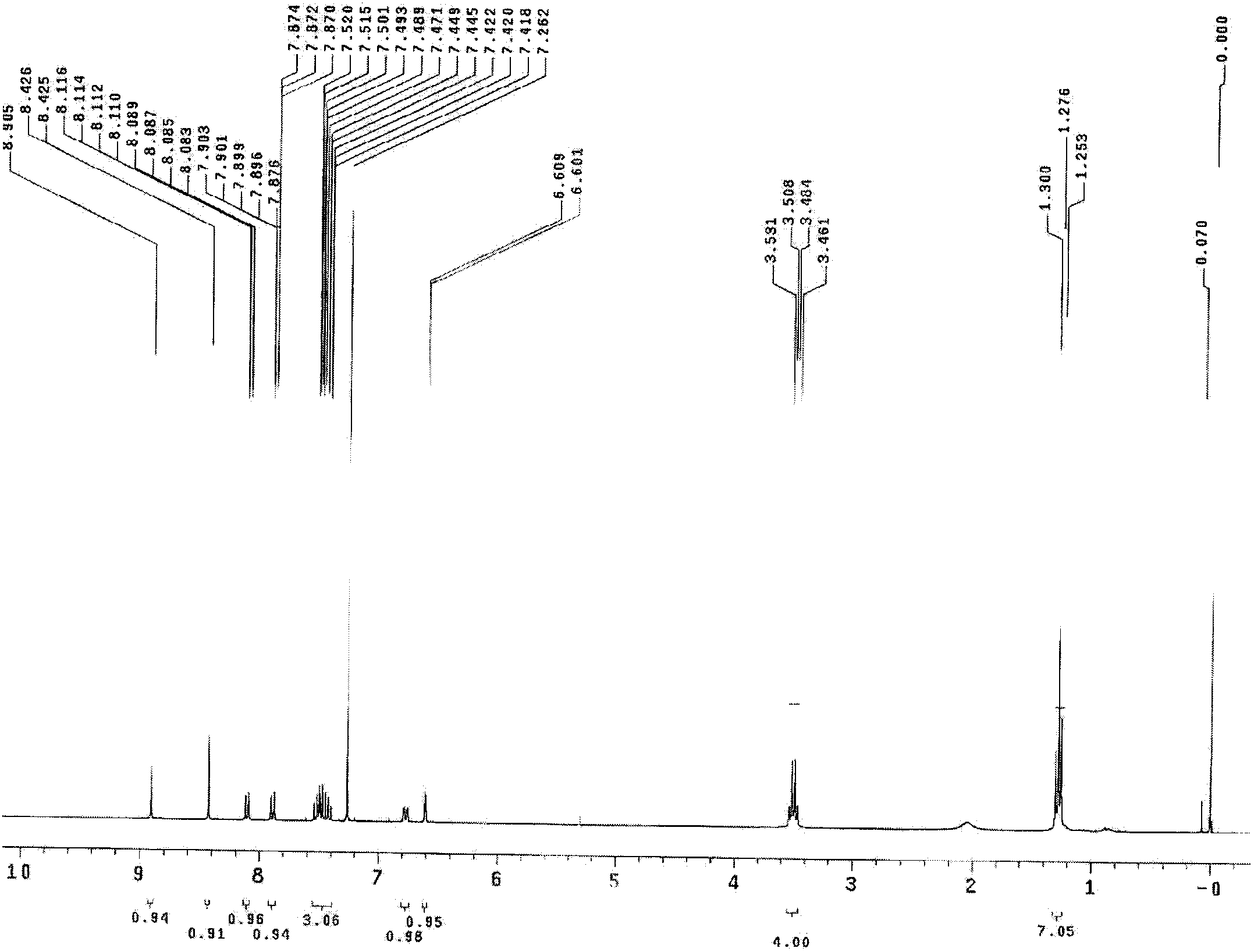
[0027] 1 HNMR (300MHz, CDCl 3 ):δ ppm =10.11 (1H, d, J4.5), 8.24 (1H, d, J4.7), 7.41 (1H, dd, J9.0, 2.8), 6.64 (1H, d, J9....
Embodiment 2
[0028] Embodiment 2: Preparation of molecular fluorescent probe
[0029] Dissolve diethylaminocoumarin aldehyde (25 mg.0.1 mmol) and benzothiazole-2-acetonitrile (18 mg.0.1 mmol) in 10 mL of absolute ethanol, add 1 drop of piperidine, and stir at room temperature for 3 hours. After the reaction, filter with suction, wash with ethanol, and separate by column chromatography (petroleum ether / dichloromethane=1:2) to obtain 28 mg (70%) of the product, which is the molecular fluorescent probe.
[0030] 1 HNMR (400MHz, DMSO-d6): δ ppm =8.91(1H, s), 8.43(1H, d, J0.4), 8.12-8.08(1H, m), 7.90-7.88(1H, m), 7.52-7.42(3H, m), 6.61(1H, s), 6.60 (1H, s), 3.49 (4H, dd, J9.4, 9.6), 1.28 (6H, d, J9.6).
Embodiment 3
[0031] Example 3: Application of naked-eye and fluorescence-enhanced detection of thiol-containing amino acid fluorescent probes
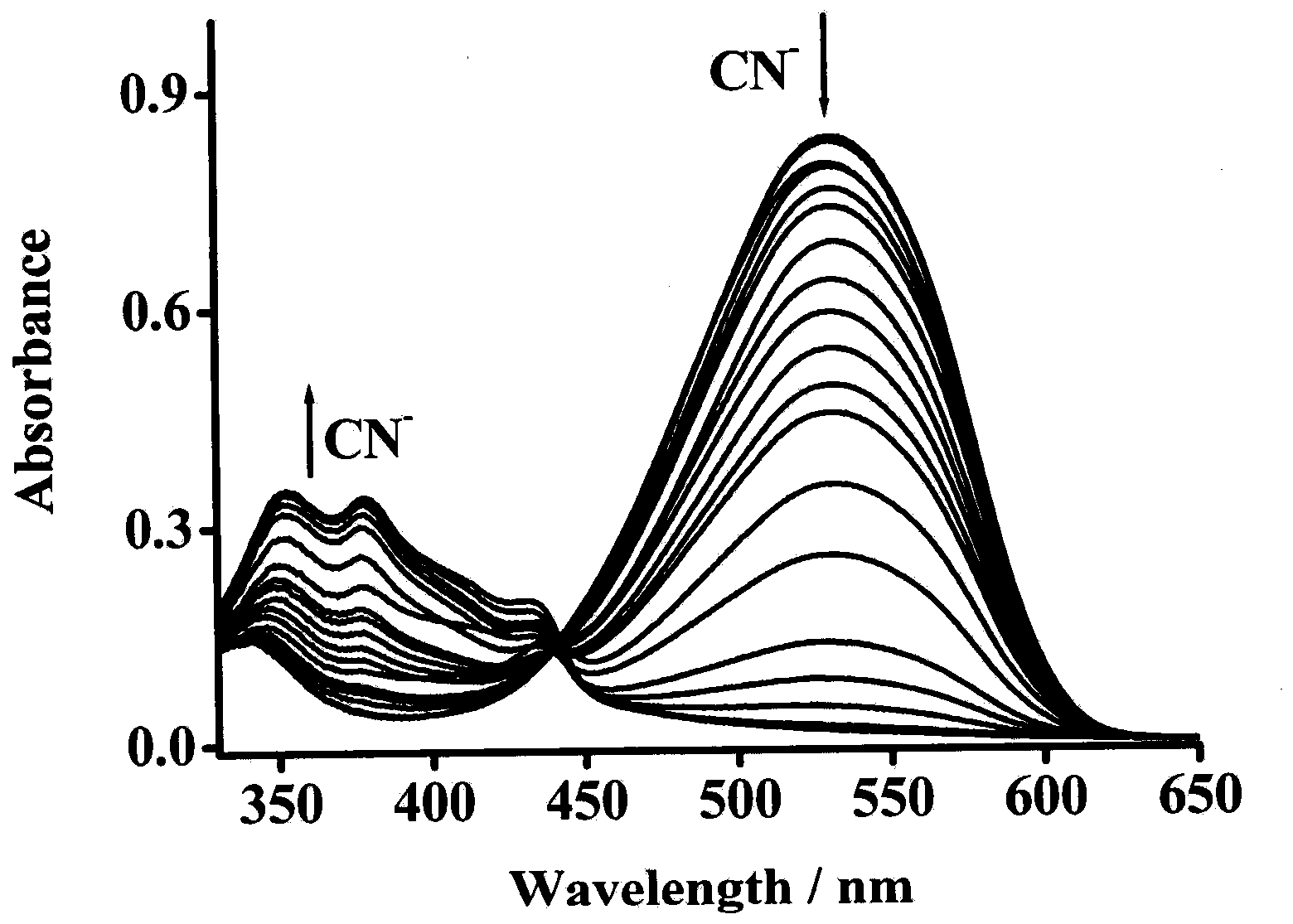
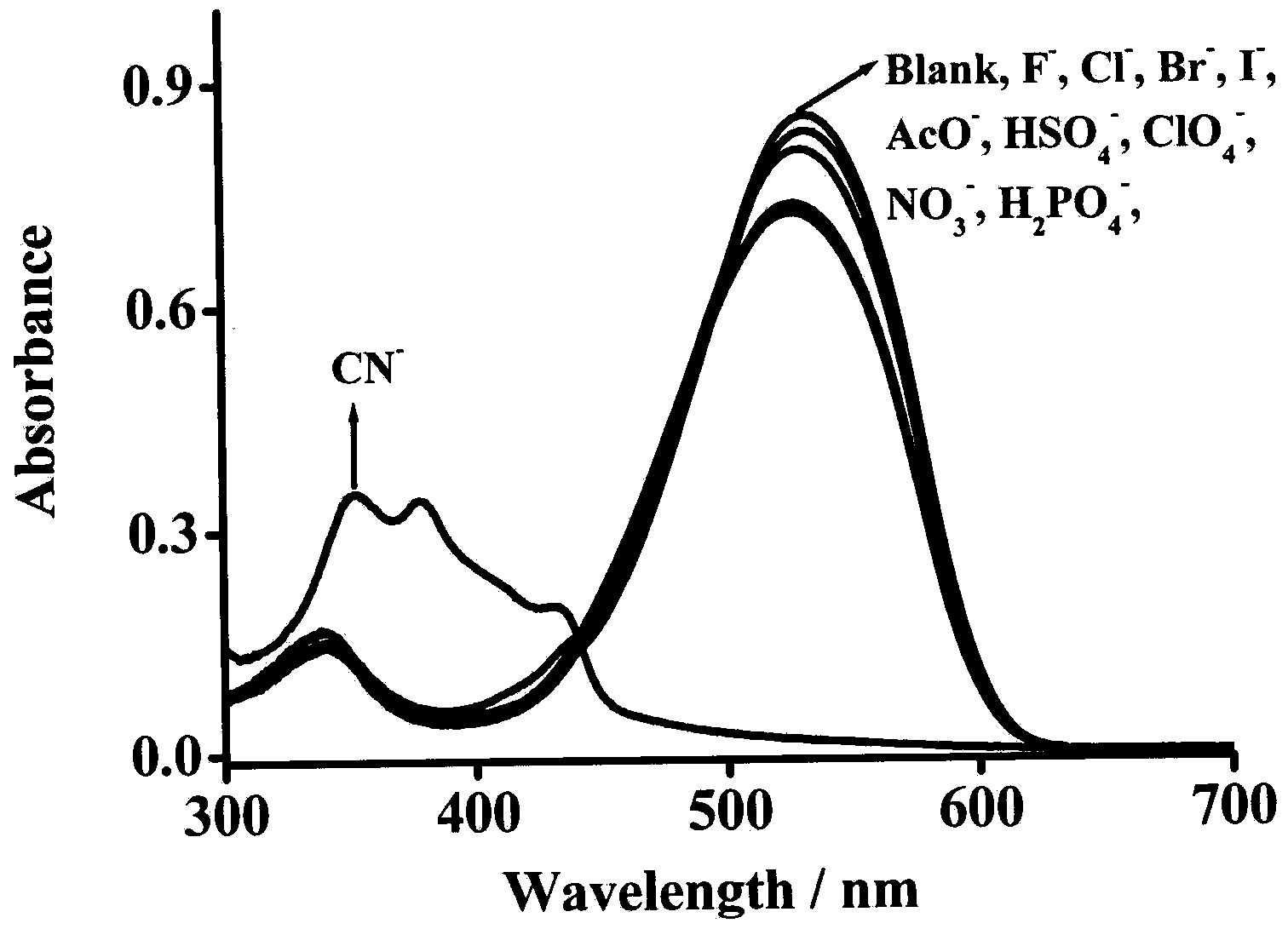
[0032] Dissolve the fluorescent probe in dimethyl sulfoxide solution to a concentration of 2.0×10 -5 mol / L and 1.0×10 -5 mol / L solution, add the corresponding anion, respectively measure the change of ultraviolet absorption spectrum and fluorescence emission spectrum. Figure 1-Figure 5 It shows that in the ultraviolet absorption spectrum and the fluorescence emission spectrum, the fluorescent probe shows high selectivity to cyanide ion, and with the increase of the concentration of cyanide ion, the ultraviolet absorption peak and the fluorescence emission peak both appear larger blue. shift, while the obvious color change is also suitable for naked eye detection. And the fluorescent probe is not affected by other anions such as: AcO - , H 2 PO 4 2- , NO 3 - , ClO 4 - , HSO 4 - , F - , Cl - , Br - , I - The effect of plasma shows th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com