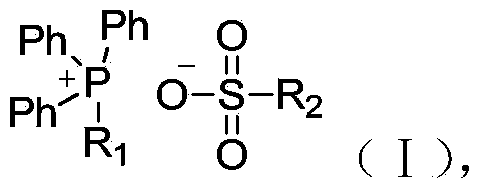Quaternary phosphonium sulfonate fire retardant and synthesis method and use thereof
A quaternary phosphonium sulfonate and a synthesis method are applied in the field of refractory materials to achieve the effects of high yield, good chemical and thermal properties, and preventing dripping
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1.1 Synthesis of sulfonate (IV) (R 1 = R 2 = )
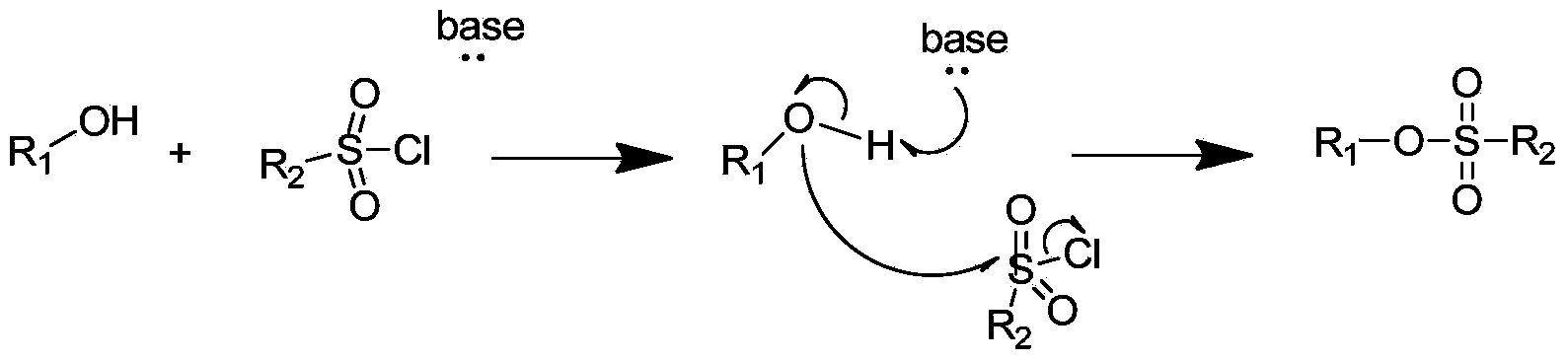
[0035] Add sodium hydride (0.12mol) to the two-necked flask, and add N 2 protective gas. Then add an appropriate amount of tetrahydrofuran. Alcohol (II) (0.1 mol) was added dropwise in an ice-water bath, and magnetically stirred for about 10 minutes at room temperature. Dissolve sulfonyl chloride (III) (0.1 mol) in an appropriate amount of tetrahydrofuran, add it dropwise to the above reaction system in an ice-water bath, and react at room temperature for about 12 hours to obtain sulfonate IV. Yield about 78%.
[0036] 1.2 Synthesis of quaternary phosphonium sulfonate flame retardant (I)
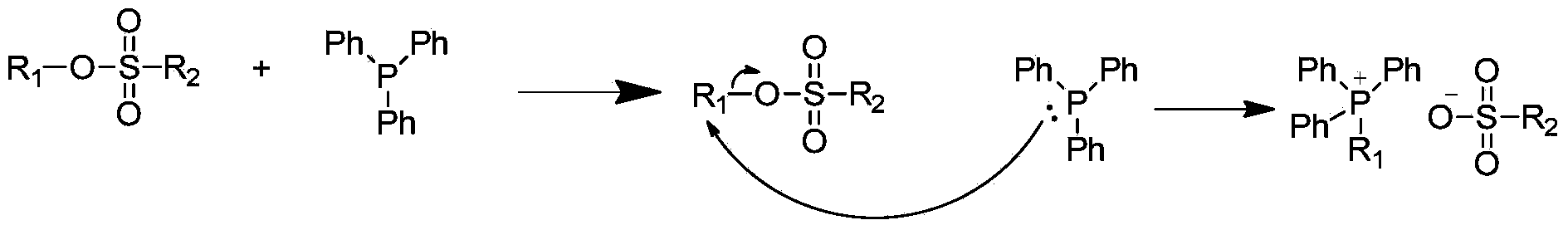
[0037] Sulfonate (IV) (0.1mol) and triphenylphosphine (V) (0.067mol) were dissolved in an appropriate amount of toluene, added to a round-bottomed flask, under N 2 Protected and heated to reflux at about 85°C for 10h. Cool to room temperature, remove toluene by rotary evaporation, wash the reaction system with diethyl ether,...
Embodiment 2
[0056] 2.1 Synthesis of sulfonate (IV) (R 1 = R 2 = )
[0057] Add sodium hydride (0.12mol) to the two-necked flask, and add N 2 protective gas. Then add an appropriate amount of tetrahydrofuran. Alcohol (II) (0.1 mol) was added dropwise in an ice-water bath, and magnetically stirred for about 10 minutes at room temperature. Sulfonyl chloride (III) (0.12mol) was dissolved in an appropriate amount of tetrahydrofuran, and added dropwise to the above reaction system in an ice-water bath, and reacted for about 12 hours at room temperature to obtain sulfonate IV. Yield about 73%.
[0058] 2.2 Synthesis of quaternary phosphonium sulfonate flame retardant (I)
[0059] Dissolve sulfonate (IV) (0.1mol) and triphenylphosphine (V) (0.1mol) in an appropriate amount of xylene, add them to a round bottom flask, and place under N 2 Protected and heated to reflux at about 140°C for 10h. Cool to room temperature, add an appropriate amount of petroleum ether to precipitate a solid, ...
Embodiment 3
[0073] 3.1 Synthesis of sulfonate (IV) (R 1 = R 2 = )
[0074] Add sodium hydride (0.12mol) to the two-necked flask, and add N 2 protective gas. Then add an appropriate amount of tetrahydrofuran. Alcohol (II) (0.1 mol) was added dropwise in an ice-water bath, and magnetically stirred for about 10 minutes at room temperature. Dissolve sulfonyl chloride III (0.15 mol) in an appropriate amount of tetrahydrofuran, add it dropwise to the above reaction system in an ice-water bath, and react for about 12 hours at room temperature to obtain sulfonate (IV). Yield about 75%.
[0075] 3.2 Synthesis of quaternary phosphonium sulfonate flame retardant (I)
[0076] Dissolve sulfonate (IV) (0.1mol) and triphenylphosphine (V) (0.1mol) in an appropriate amount of xylene, add them to a round bottom flask, and place under N 2 Protected and heated to reflux at about 145°C for 12 hours. Cool to room temperature, add an appropriate amount of petroleum ether to precipitate a solid, let ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melt index | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com