Method and system for removing sulfur dioxide in flue gas
A sulfur dioxide and flue gas technology, applied in chemical instruments and methods, separation methods, preparation of alkali metal sulfites, etc., can solve the problems of high operating cost and low desulfurization efficiency, and achieve low operating cost, long contact time, The effect of reducing operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
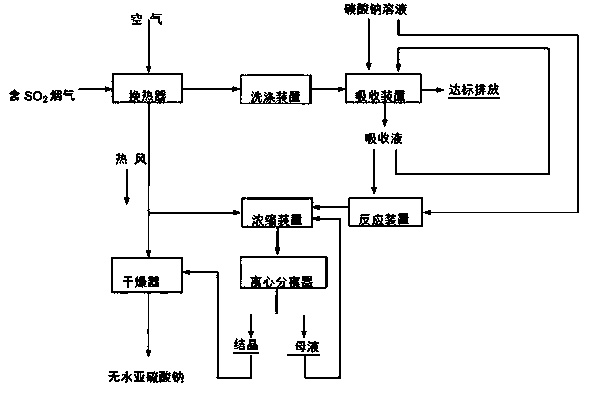
[0059] 1), the pending SO 2 The concentration is 35000mg / Nm 3 1. Flue gas from a zinc smelting rotary kiln at a temperature of 280°C and air at normal temperature are respectively passed into heat exchanger 1 for heat exchange, so that the temperature of the flue gas is reduced to 160°C, and at the same time, the air is heated into hot air at 110°C;
[0060] 2) The sulfur-containing flue gas after heat exchange is sent to the washing tower for washing to remove the soot and SO in the flue gas 3 and other impurities, and further reduce the flue gas temperature to 60°C;
[0061] 3) The sulfur-containing flue gas after purification and cooling is successively sent to the tubular rotary shear flow absorber and the spray absorption tower to be mixed with 35% sodium carbonate, 15% sodium sulfite, and 5% sodium bisulfite Solution reverse contact reaction, sulfur-containing flue gas is absorbed to form clean flue gas emission, and generates Na 2 SO 3 and NaHSO 3 the m...
Embodiment 2
[0067] 1) The SO2 concentration to be treated is 20000 mg / Nm 3 1. Flue gas from a zinc smelting volatilization kiln at a temperature of 250°C and air at normal temperature are respectively passed into heat exchanger 1 for heat exchange, so that the temperature of the flue gas is reduced to 150°C, and at the same time, the air is heated into hot air at 110°C;
[0068] 2) The sulfur-containing flue gas after heat exchange is sent to the washing tower for washing to remove the soot and SO in the flue gas 3 and other impurities, and further reduce the flue gas temperature to 70°C;
[0069]3) The sulfur-containing flue gas after purification and cooling is successively sent to the tubular rotary shear flow absorber and the spray absorption tower to be mixed with 40% sodium carbonate, 18% sodium sulfite, and 8% sodium bisulfite Solution reverse contact reaction, sulfur-containing flue gas is absorbed to form clean flue gas emission, and generates Na 2 SO 3 and NaHSO 3 the...
Embodiment 3
[0075] 1) will be treated containing SO 2 Concentration 40000 mg / Nm 3 1. Flue gas from a zinc smelting volatilization kiln at a temperature of 250°C and air at normal temperature are respectively passed into heat exchanger 1 for heat exchange, so that the temperature of the flue gas is reduced to 180°C, and at the same time, the air is heated into hot air at 120°C;
[0076] 2) The sulfur-containing flue gas after heat exchange is sent to the washing tower for washing to remove the soot and SO in the flue gas 3 and other impurities, and further reduce the flue gas temperature to 65°C;
[0077] 3) The sulfur-containing flue gas after purification and cooling is successively sent to the tubular rotary shear flow absorber and the spray absorption tower to be mixed with 40% sodium carbonate, 20% sodium sulfite, and 10% sodium bisulfite Solution reverse contact reaction, sulfur-containing flue gas is absorbed to form clean flue gas emission, and generates Na 2 SO 3 and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com