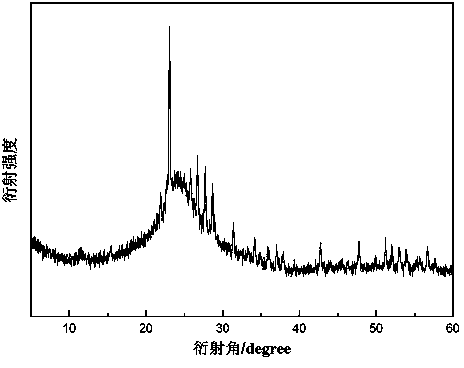
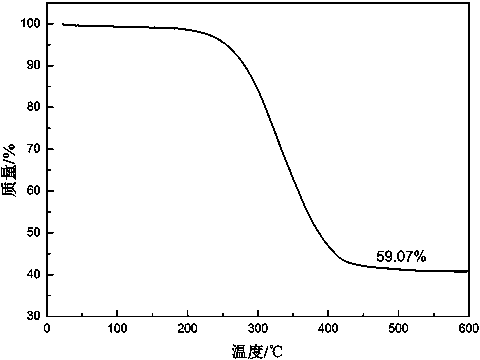
A preparation method of a sulfur-supported graphene aerogel composite material
A technology of graphene airgel and composite materials, which is applied in the field of preparation of graphene airgel-supported sulfur composite materials, and can solve the problems of poor cycle stability of lithium-sulfur batteries, poor cycle stability of batteries, and reduced utilization of sulfur and other problems, to achieve the effect of short preparation cycle, improved electrochemical performance and large output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] One, prepare graphene oxide with traditional known process
[0033] Potassium persulfate (K 2 S 2 o 8 ) 2.5 g, phosphorus pentoxide (P 2 o 5 ) 2.5 g, dissolved in 12 mL of concentrated sulfuric acid, heated to 80°C; then 3 g of natural graphite was added to the above solution, kept at 80°C for 4.5 hours; cooled to room temperature, diluted with 500 mL of deionized water, and left standing overnight ; filter, float residual acid with 0.2 mm filter; dry in a vacuum oven at 60°C; add the obtained preoxide to 120 mL of ice-bathed concentrated sulfuric acid, slowly add 15 g of KMnO under stirring 4 , Keep the temperature below 20°C during the addition process. Then the temperature was controlled at 35°C and stirred for 2 h. Add 250 mL of deionized water to dilute, and keep the temperature below 50°C in an ice bath during the dilution process. Stir for another 2 h, add 0.7 L of deionized water, and immediately add 20 mL of 30% H 2 o 2 , the mixture produced bubbles, ...
Embodiment 2
[0042] The preparation method of graphene oxide is the same as above-mentioned embodiment 1.
[0043] 1) Disperse 80 mg of graphene oxide in 50 mL of deionized water, sonicate for 24 h; add 5 mL of isopropanol solution, stir for 5 h; add 3 mL of sulfur / carbon disulfide solution with a mass-volume ratio of 75 mg / mL , stirred for 1.5 h; the solution was transferred to a reaction kettle, kept at a constant temperature for 48 h at 100 °C, soaked in alcohol and deionized water three times, frozen and sectioned, and dried to obtain a composite material of graphene airgel loaded with sulfur ;
[0044] 2) Immerse the graphene airgel composite material in a solution of 0.1 mol / L pyrrole monomer and 1 mol / L hydrochloric acid for 0.5 h; place the soaked material on a watch glass, add potassium permanganate dropwise, and react After 24 h, the polypyrrole-coated graphene airgel-loaded sulfur composite was obtained.
Embodiment 3
[0046] The preparation of graphene oxide is the same as above-mentioned embodiment 1.
[0047] 1) Disperse 200 mg of graphene oxide in 50 mL of deionized water, sonicate for 12 h; add 25 mL of absolute ethanol solution, stir for 2 h; add 3 mL of sulfur / carbon disulfide solution with a mass-volume ratio of 100 mg / mL , stirred for 1.5 h; the solution was transferred to the reactor, kept at a constant temperature of 150 °C for 24 h, soaked in alcohol and deionized water three times respectively, frozen and sectioned, and dried to obtain a composite material of graphene airgel loaded with sulfur ;
[0048] 2) Immerse the graphene airgel composite material in a solution of 0.5 mol / L aniline monomer and 1 mol / L hydrochloric acid for 0.5 h; place the soaked material on a watch glass, add potassium dichromate dropwise, and react for 24 h Obtained polyaniline-coated graphene airgel-loaded sulfur composites.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com