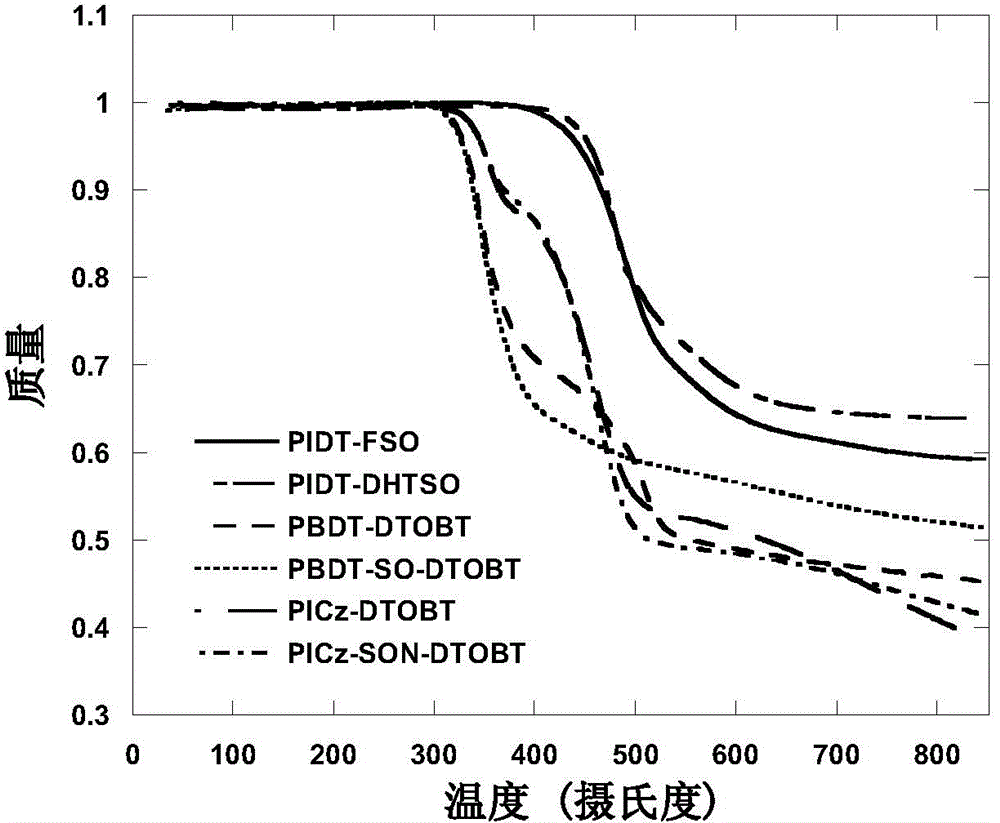
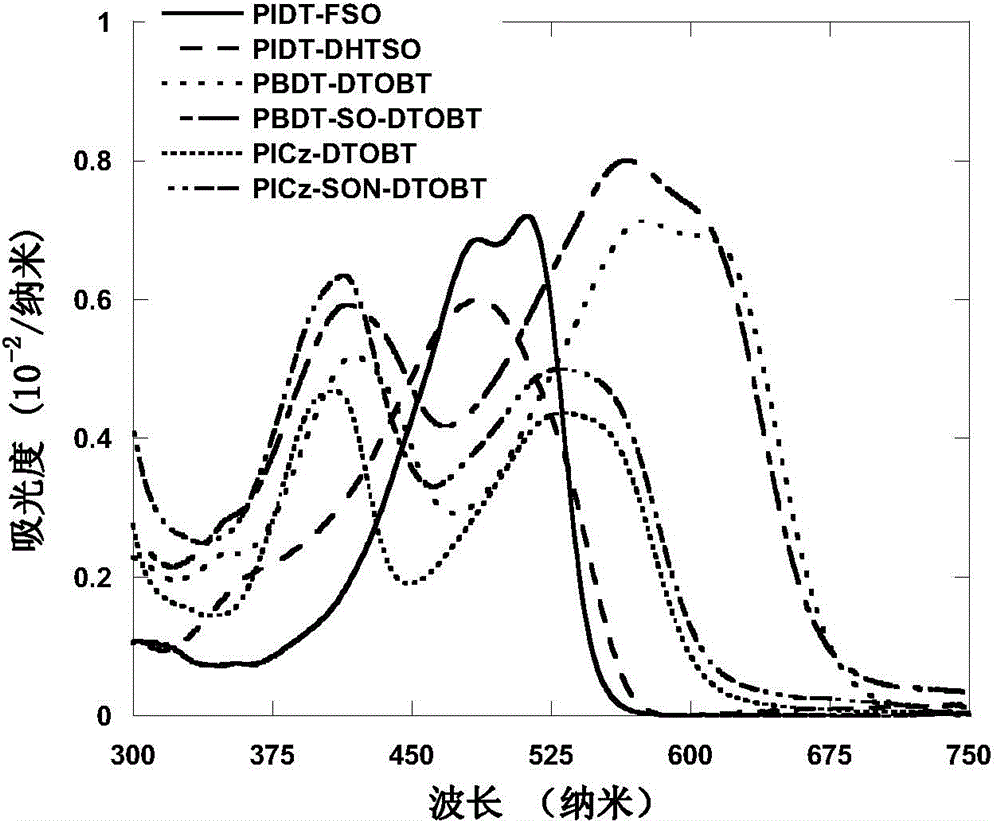
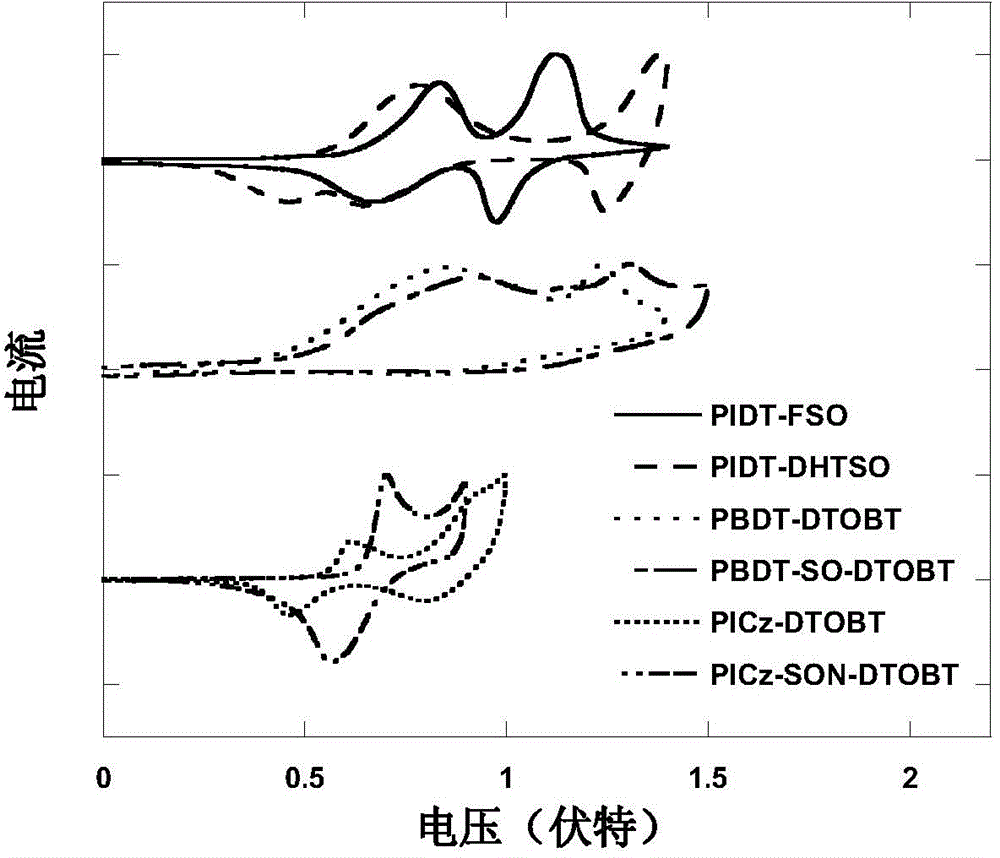
S,S-dioxo-dibenzothiophene unit-containing electron donor polymer and use thereof
A technology of dibenzothiophene and electron donor, applied in electric solid devices, circuits, photovoltaic power generation, etc., can solve problems such as difficulties, and achieve the effects of good solubility, deep HOMO energy level, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation route of 3,7-dibromo-S,S-dioxo-dibenzothiophene
[0025] (1) S, the preparation of S-dioxy-dibenzothiophene, the reaction formula is as follows:
[0026]
[0027] In a 500ml three-neck round bottom flask, add dibenzothiophene (18.4g, 100mmol), acetic acid (200ml) and hydrogen peroxide (50mL), heat and reflux for overnight reaction, after the reaction, the reaction temperature drops to room temperature, and a large amount of crystals are precipitated from the reaction solution , suction filtration, and successively wash the filter residue several times with acetic acid and ethanol. After drying, 17.3 g of colorless needle-like crystals were obtained, yield: 80%. (2) Preparation of 3,7-dibromo-S, S-dioxy-dibenzothiophene, the reaction formula is as follows:
[0028]
[0029] In a 500ml single-necked flask, add S, S-dioxy-dibenzothiophene (10.8g, 50mmol), concentrated sulfuric acid (150ml), after stirring and dissolving, slowly add N-bromosuccinimide ( ...
Embodiment 2
[0031] Preparation route of 2,8-difluoro-3,7-dibromo-S,S-dioxo-dibenzothiophene
[0032] (1) The preparation of 2,8-difluoro-S, S-dioxy-dibenzothiophene, the reaction formula is as follows:
[0033]
[0034] In a 500ml three-neck round bottom flask, add 4,4'-difluorodiphenyl sulfone (15.2g, 60mmol), ketone chloride (20.2g, 150mmol) and anhydrous tetrahydrofuran (THF) (300ml), and stir to cool down to After -78°C, n-butyllithium solution (2.5M in THF, 53mL, 132mmol) was added dropwise. After the dropwise addition, the reaction was slowly heated to 20°C and reacted for another 12 hours, then quenched with ammonium chloride aqueous solution. Filter, wash the filter residue with water and ethanol successively, after drying, recrystallize with chlorobenzene. 4 g of white crystals were obtained, yield: 25%.
[0035] (2) Preparation of 2,8-difluoro-3,7-dibromo-S,S-dioxo-dibenzothiophene, the reaction formula is as follows:
[0036]
[0037] In a 100ml three-neck round bottom...
Embodiment 3
[0039] The preparation route of 3,7-dibromo-2,8-bis(3′-(N,N-diethylamino)propoxy)-S,S-dioxy-dibenzothiophene:
[0040] (1) The preparation of 2,8-dibromo-dibenzothiophene, the reaction formula is as follows:
[0041]
[0042] In a 1000 ml three-necked flask, add dibenzothiophene (36.8 g, 200 mmol), iron powder (0.6 g, 10 mmol) and chloroform (500 mL). Stir until dissolved, add bromine (80g, 500mmol) dropwise under ice bath, after the dropwise addition is complete, react in the dark at room temperature for 12 hours, after the reaction is complete, add aqueous sodium bisulfite solution to the reaction solution until the solution turns white, filter, and filter the residue After washing several times with water and methanol successively, after drying, it was recrystallized with tetrahydrofuran to obtain 48 g of white solid, yield: 70%.
[0043] (2) Preparation of 2,8-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-dibenzothiophene, the reaction formula is as follows:
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com