Carbonate polymer with disulfur five-membered ring functional group on side chain and application thereof
A carbonate polymer and functional group technology, applied in the field of medical materials, can solve the problems of cumbersome preparation process and achieve the effects of simple preparation, excellent biodegradability, and simplified operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Synthesis of a cyclic carbonate monomer (CDC) containing a disulfide five-membered ring functional group
[0053]
[0054] 1. Sodium hydrosulfide monohydrate (28.25g, 381.7mmol) was dissolved in 400mL N,N-dimethylformamide (DMF), heated at 50°C until completely dissolved, and dibromoneopentyl glycol (20g, 76.4mmol), reacted for 48 hours. The reactant was distilled off under reduced pressure to remove the solvent DMF, then diluted with 200mL distilled water, extracted four times with 250mL ethyl acetate, and finally the organic phase was rotary evaporated to obtain yellow viscous compound A, yield: 70%;
[0055] 2. Compound A dissolved in 400 mL of tetrahydrofuran (THF) was placed in the air for 24 hours, and the intermolecular sulfhydryl groups were oxidized into sulfur-sulfur bonds to obtain Compound B, with a yield of >98%;
[0056] 3. Under nitrogen protection, compound B (11.7 g, 70.5 mmol) was dissolved in dried THF (150 mL), and stirred until complet...
Embodiment 2
[0057] Example 2 Synthesis of carbonate polymer PEG5k-P (CDC2.5k-co-CL3.9k) containing disulfide five-membered ring functional groups in two-block side chains
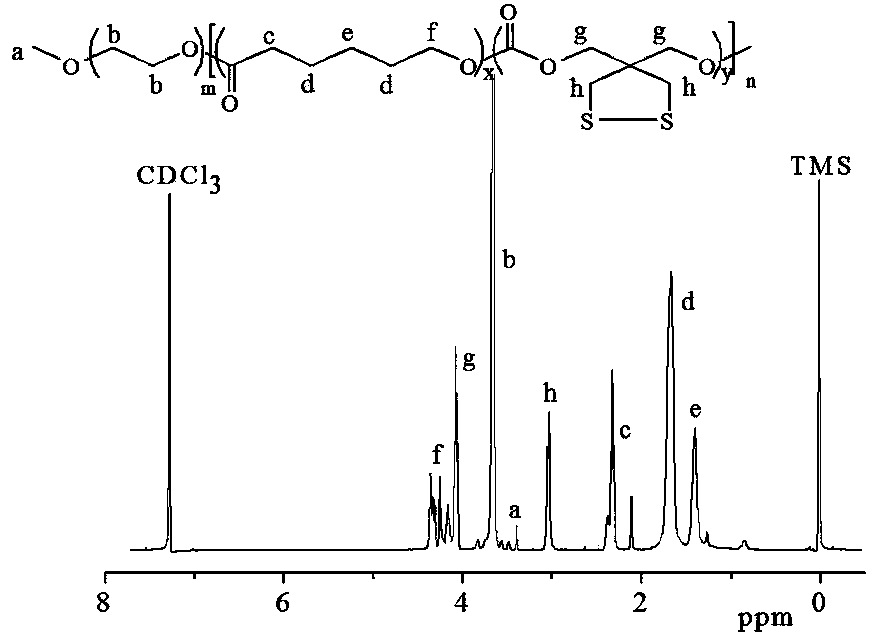
[0058] Under a nitrogen atmosphere, 0.28g (1.46mmol) of CDC monomer and 0.4g (3.51mmol) of caprolactone (ε-CL) were dissolved in 3mL of dichloromethane and added to the sealed reactor, and then polystyrene with a molecular weight of 5000 was added. Ethylene glycol 0.5g (0.1mmol) and the dichloromethane solution (0.1mol / L) of the catalyzer bis(bistrimethylsilyl)amine zinc of 0.1mol / L, then seal the reactor well, transfer out the glove box , placed in a 40°C oil bath to react for 1 day, terminate the reaction with glacial acetic acid, precipitate in glacial ether, and finally filter and vacuum-dry to obtain a carbonate polymer PEG5k- P(CDC2.5k-co-CL3.9k).
[0059]
[0060] In the formula, m=113.6, x=34.2, y=13.0, n=47.2.
[0061] attached figure 1 is the NMR spectrum of the above polymer. 1 H NMR (400MHz, CDCl 3 ...
Embodiment 3
[0062] Example 3 Synthesis of Carbonate Polymer PEG5k-b-PCDC2.8k Containing Disulfide Five-membered Ring Functional Groups in Two Block Side Chains
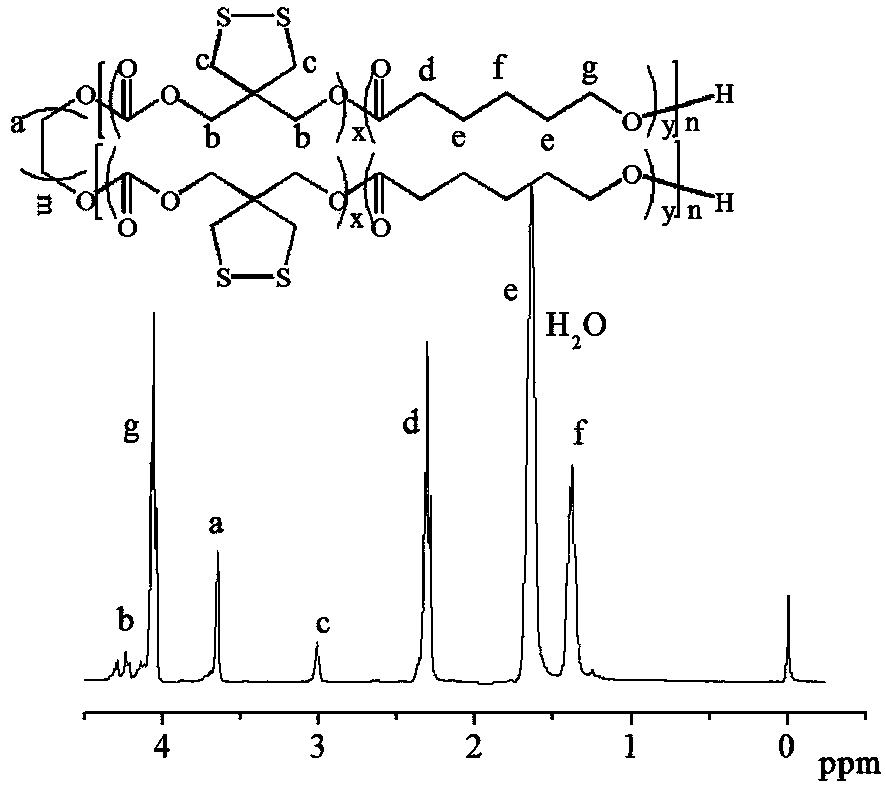
[0063] Under nitrogen atmosphere, add 0.3g (1.56mmol) of CDC monomer, 2mL of dichloromethane into the sealed reactor, then add 0.5g (0.1mmol) of polyethylene glycol with a molecular weight of 5000 and 1mL of catalyst bis(bistri Methylsilyl) amine zinc dichloromethane solution (0.1mol / L), then seal the reactor well, transfer it out of the glove box, put it into a 40°C oil bath and react for 1 day, terminate the reaction with glacial acetic acid, and Precipitate in diethyl ether, and finally filter and vacuum-dry to obtain a carbonate polymer PEG5k-b-PCDC2.8k containing a disulfide five-membered ring functional group in the side chain of the product.
[0064] 1 H NMR (400MHz, CDCl 3 ): 3.08 (s, -CCH 2 ), 3.30 (m, -OCH 3 ), 4.05(s, -CH 2 OCOCHCH 2 -), 4.07(s, -OCH 2 CCH 2 O-), 4.31 (m, -CCH 2 ).
[0065]
[0066] In the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com