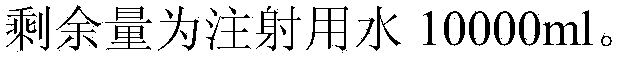Antimicrobial peptide freeze-dried preparation for treating colpitis and preparation method thereof
A freeze-dried preparation and antibacterial peptide technology, applied in the medical field, can solve the problems of insignificant drug treatment effect, incomplete treatment, bacterial resistance, etc., and achieve the effect of accelerating wound healing process, protecting physical and mental health, and strong killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The freeze-dried powder is configured as follows: Weigh 50g of antimicrobial peptide human beta defensin, 5g of low molecular weight heparin sodium, 600g of stabilizer, add 10000ml of injection grade water, mix well, filter and sterilize, fill in 3.2ml of vials and half-stopper Then put it into a vacuum freeze dryer and freeze-dry for 15 hours. The process conditions of vacuum freeze-drying are as follows: pre-freeze the product, lower the temperature to -50°C, cool down for 4 hours, evacuate to 0.3mbar, heat and sublimate and dry for 15 hours, until the water in the product is completely sublimated .
[0031] Wherein the stabilizer formula is: 200g glycine, 300g protein hydrolyzate, 100g (10mM) phosphoric acid.
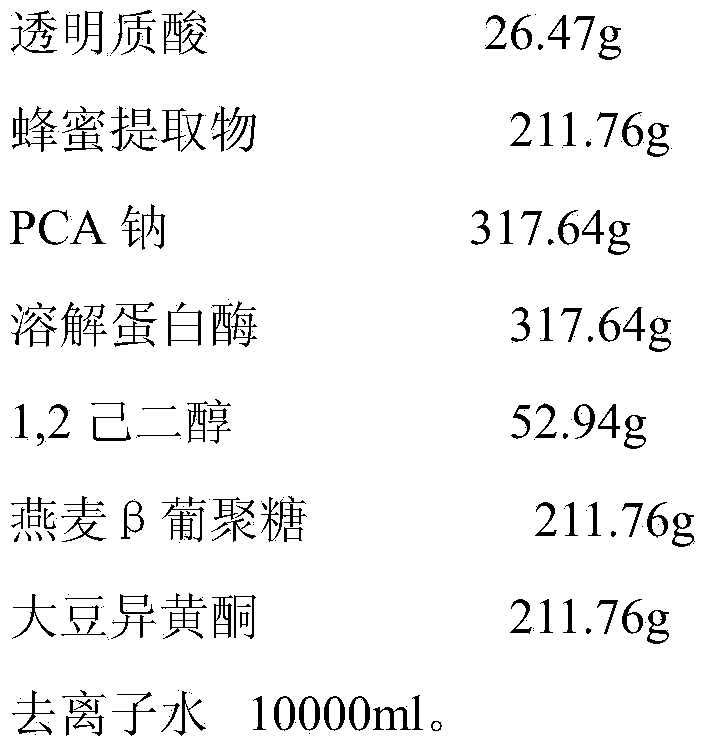
[0032] The configuration of basic liquid of the present invention is:
[0033] Basic liquid formula:
[0034]
[0035]
[0036] After the above formula is dissolved, it is sterilized by filtration, filled and capped in 15ml / bottle. When in use, the a...
Embodiment 2
[0038] Freeze-dried powder of the present invention is configured as:
[0039] Weigh 80g of antimicrobial peptide human beta defensin, 8g of low molecular weight heparin sodium, 500g of stabilizer, add 10000ml of water for injection, mix evenly, filter and sterilize, fill in 3.2ml of vials with a half-stopper and put them into vacuum freeze-drying Freeze-dried on the machine for 25 hours. The process conditions of vacuum freeze-drying are: pre-freeze the product, lower the temperature to -50°C, cool down for 6 hours, vacuumize to 0.3mbar, heat and sublimate and dry for 20 hours, until the water in the product sublimates completely .
[0040] Wherein the stabilizer formula is: 150g trehalose, 200g sodium thiosulfate, 150g (15mM) sodium carbonate
[0041] The configuration of basic liquid in the present embodiment is:
[0042] Basic liquid formula:
[0043]
[0044] After the above formula is dissolved, it is sterilized by filtration, filled and capped in 15ml / bottle. Wh...
Embodiment 3
[0046] Freeze-dried powder of the present invention is configured as:
[0047] Weigh 100g of antimicrobial peptide, 5g of low molecular weight heparin sodium, 500g of stabilizer, add 10000ml of water for injection, mix evenly, filter and sterilize, fill in 3.2ml in a vial and put it in a vacuum freeze dryer for 35 hours with a stopper. . The process conditions of vacuum freeze-drying are: pre-freeze the product, lower the temperature to -50°C, cool down for 8 hours, vacuumize to 0.3mbar, heat and sublimate and dry for 25 hours, until the water in the product sublimates completely .
[0048] Wherein the stabilizer formula is: 200g sucrose, 200g vitamin D, 100g (10mM) potassium citrate
[0049] The configuration of the basic liquid in this embodiment is:
[0050] Basic liquid formula:
[0051]
[0052]After the above formula is dissolved, it is sterilized by filtration, filled and capped in 15ml / bottle. When in use, the antimicrobial peptide freeze-dried powder of the pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com