Antibacterial peptide as well as applications thereof to preparation of anti-infective drugs, antitumor drugs, immunopotentiators and feed additives
An anti-tumor drug and antibacterial peptide technology, applied in anti-tumor drugs, animal feed, antibacterial drugs, etc., can solve the problems of low natural yield of antibacterial peptides, expensive chemical synthesis, complicated natural extraction steps, etc., and achieve significant growth inhibition. effect, promote the secretion of IFNγ, and improve the effect of immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1. Gene Cloning of Plutella xylostella antimicrobial peptide pxCA1
[0056] 1.1 Infection immunity of diamondback moth larvae
[0057] The larvae of Plutella xylostella were isolated and artificially raised to 4-7 days old. Escherichia coli JM109 cultured to the logarithmic phase was dipped in a needle to perform stress puncture on the abdomen of Plutella xylostella. After 24 hours of continuous feeding, the total RNA of the larvae was extracted.
[0058] 1.2 Extraction of total RNA
[0059] ① Grind the tissue specimen in a small grinder pre-cooled at -80°C, add Trizol reagent to the grinder, add 1ml TRIzol reagent per 50-100 mg of tissue (the volume of the tissue block should not exceed 10% of the volume of TRIzol), and fully grind the tissue into a homogenate; use a pipette gun to draw the homogenate into a 1.5ml EP tube, and place it on ice for 5 minutes.
[0060] ②After the nucleic acid and protein are fully dissociated, add 0.2ml chloroform to each 1ml TR...
Embodiment 2
[0083] Example 2 Expression and Extraction of Diamondback Moth Antimicrobial Peptide pxCA1 in Prokaryotic
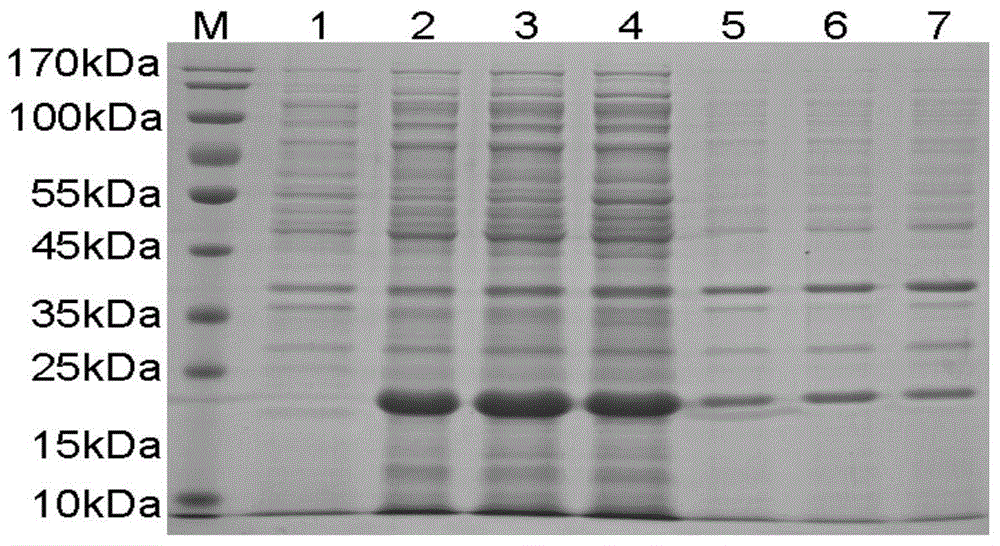
[0084] 2.1 Construction and induced expression of genetic engineering fusion antimicrobial peptide prokaryotic expression engineering bacteria
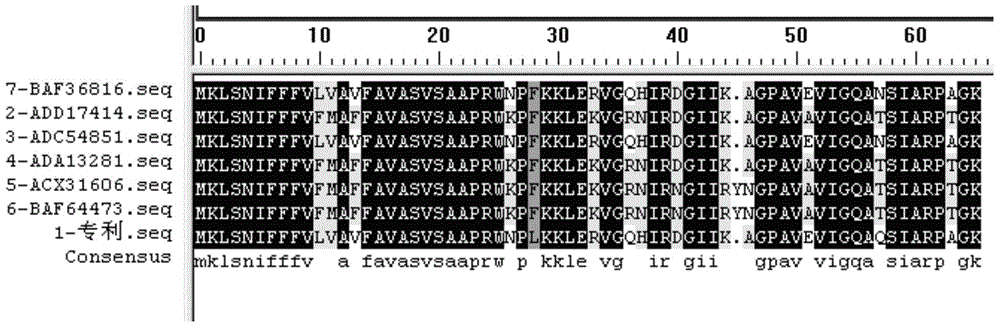
[0085]According to the analysis of the online signal peptide prediction server and referring to the literature on the post-translational modification of cecropin family antimicrobial peptides, the amino acids in the signal peptide and N-terminal immature peptides in the full-length peptide (SEQ ID No.2) expressed by the pxCA1 gene were removed. Codon optimization was performed on the coding sequence of the mature peptide (25-65 amino acids) (SEQ ID No.3), and the coding gene suitable for expression in Escherichia coli was synthesized as shown in SEQ ID No.4.
[0086] The coding gene was constructed into the pET32a plasmid, the upstream and downstream cloning sites were KpnI / XhoI, and an enterokinase cleavage site (DDDDK▼) was in...
Embodiment 3
[0102] The mensuration (MTT method) of embodiment 3.pxCA1 antimicrobial peptide minimum antibacterial concentration
[0103] Escherichia coli (K12D31), Staphylococcus aureus (CMCC26003), Bacillus aeruginosa (CMCC10104), Bacillus subtilis (CMCC63501) and methicillin-resistant Staphylococcus aureus (MRSA5636) were cultured in LB liquid until OD 630 =0.6, and were diluted to 10 with LB 5 -2×10 5 CFU / ml.
[0104] Dilute the purified and concentrated pxCA1 antimicrobial peptide solution with LB. Take 10 μl of each dilution of the antimicrobial peptide solution and mix it with 90 μl of the diluted bacterial suspension; after culturing at 37°C for 6 hours, take Add 20 μl to a 96-well plate; add 10 μl of MTT to each well, incubate at 37°C for 1 hour, add 90 μl DMSO to each well to dissolve the insoluble matter formed in the well, and measure the absorbance of each well at 570 nm on a microplate reader. After that, 20 μl of the mixed culture solution was repeatedly sampled every 2 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com