Cholesterol-poloxamer-cholesterol triblock copolymer, preparation method and application thereof
A cholesterol and tri-block technology, which is applied in the field of preparation of poloxamer derivatives, can solve the complex synthesis and purification process of unilaterally grafted cholesterol, the insignificant reduction of critical micelle concentration, and the short cycle time of anhydride-ester bonds, etc. problem, to achieve the effect of improving drug loading capacity, low critical micelle concentration, and improving dilution stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
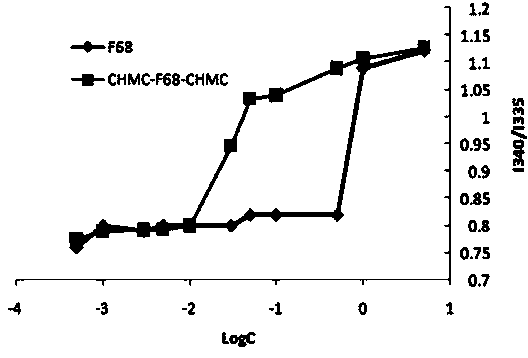
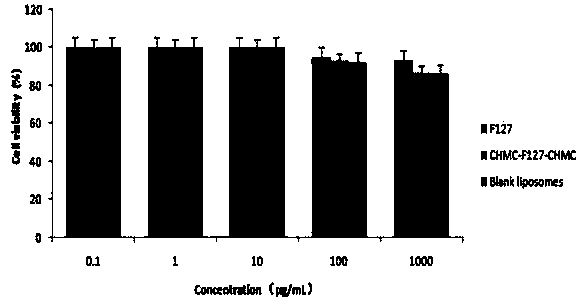
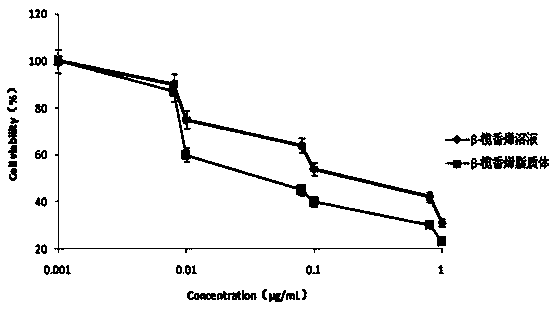
[0056] Take 336 mg of F68 (0.04 mM) in a closed container, add 2.44 mg of 4-lutidine and 20 μL of triethylamine under nitrogen, slowly add a solution of cholesteryl chloromethyl ester (0.12 mM) in dichloromethane 8 mL, stirred and mixed in an ice-water bath for 30 min, then reacted at room temperature for 24 h, and removed the solvent under reduced pressure after the reaction was completed. A certain amount of distilled water was added to the obtained crude product, extracted three times with dichloromethane, washed three times with ice water, saturated sodium chloride and 100 mM hydrochloric acid, and precipitated with ice ether to obtain a white wax, which was further refined by repeated precipitation for 3 The CHMC-F68-CHMC three-block copolymer is obtained in one time.
[0057] During the reaction, silica gel thin-layer chromatography (TLC) was used to monitor the progress of the reaction and analyze the purity. Developing agent: chloroform: methanol: water: acetone: glac...
Embodiment 2
[0062] Take 504 mg F127 (0.04 mM) in a closed container, add 2.44 mg 4-lutidine and 20 μL triethylamine under nitrogen, slowly add a dichloromethane solution of cholesteryl chloromethyl ester (0.12 mM) dropwise8 mL, stirred and mixed in an ice-water bath for 30 min, then reacted at room temperature for 24 h, and removed the solvent under reduced pressure after the reaction was completed. Add a certain amount of distilled water to the obtained crude product, extract three times with dichloromethane, wash three times with ice water, saturated sodium chloride and 100 mM hydrochloric acid respectively, and precipitate a white waxy substance through ice ether precipitation, continue to repeatedly precipitate and refine Three times to get powdered CHMC-F127-CHMC tri-block copolymer.
[0063] During the reaction, silica gel thin-layer chromatography (TLC) was used to monitor the progress of the reaction and analyze the purity. Developing agent: chloroform: methanol: water: acetone: ...
Embodiment 3
[0068] Take 308 mg F87 (0.04 mM) and place it in a closed container, add 2.44 mg 4-lutidine and 20 μL triethylamine under nitrogen, slowly add cholesteryl chloromethyl ester (0.12 mM) in dichloromethane solution 8 mL, stirred and mixed in an ice-water bath for 30 min, then reacted at room temperature for 24 h, and removed the solvent under reduced pressure after the reaction was completed. Add a certain amount of distilled water to the obtained crude product, extract three times with dichloromethane, wash three times with ice water, saturated sodium chloride and 100 mM hydrochloric acid respectively, and precipitate a white waxy substance through ice ether precipitation, continue to repeatedly precipitate and refine Three times to get powdered CHMC-F87-CHMC tri-block copolymer.
[0069] Use IFS-55 Fourier Transform Infrared Spectrometer (Bruker Company, Switzerland) to carry out infrared analysis on the product obtained, and the test spectrum is shown in the accompanying drawi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Final concentration | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com