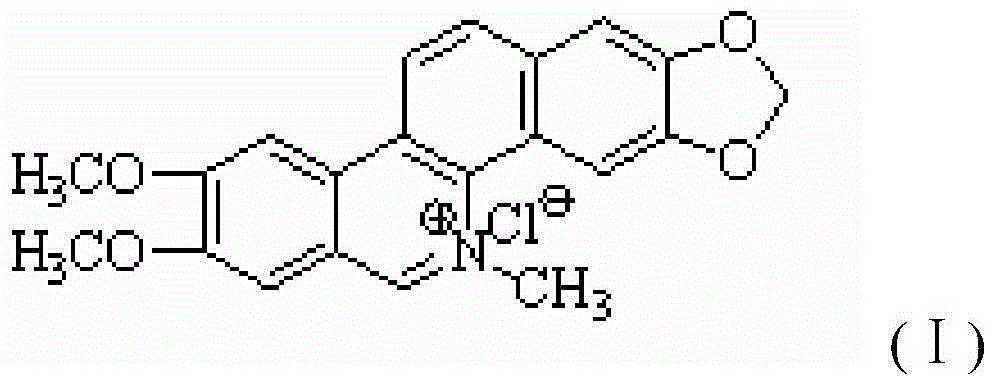
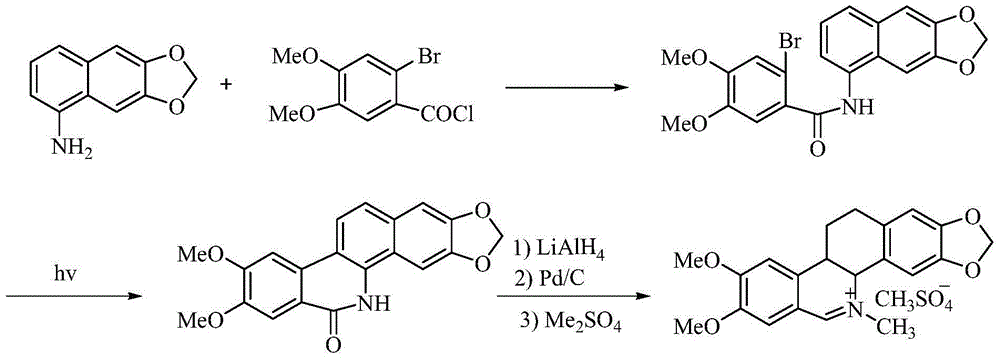
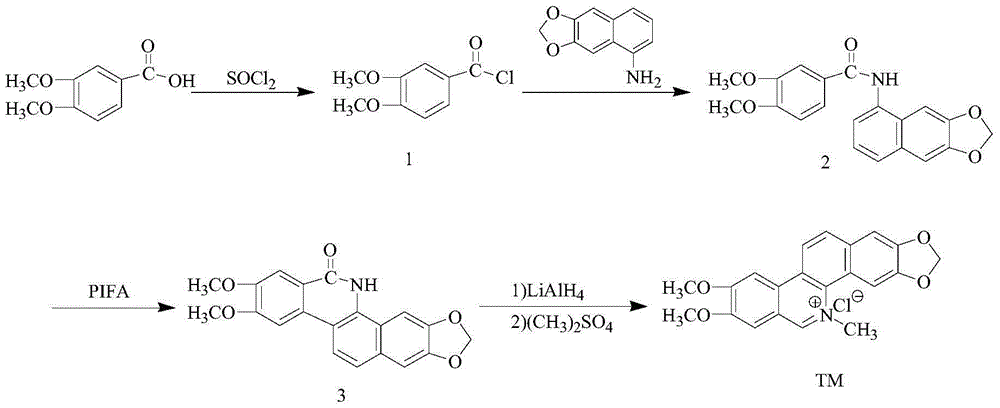
Synthetic method of nitidine chloride
A technology of chlorinated syringamine and a synthesis method, applied in the field of medicine, can solve the problems of complicated operation, many side reactions, unfavorable industrial production and the like, and achieves the effect of simple synthesis route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) Synthesis of intermediate 1:
[0026] Dissolve 0.98g of 3,4-dimethoxybenzoic acid (5.4mmol) in 40mL of dichloromethane, then add 0.78mL of SOCl 2 (10.8mmol) was heated to reflux at 40°C for 4h. The solvent was evaporated to dryness to obtain a white solid, namely intermediate 1, with a yield of 100%.
[0027] 2) Synthesis of Intermediate 2:
[0028] Take the above 1.12g of intermediate 1 and dissolve it in 40mL of dichloromethane, add 2.8mL of pyridine and 1g of 3,4-methylenedioxyaniline to it at 0°C (3,4-methylenedioxyaniline is divided into 3 batches within 10min added), and then reacted at 5°C for 24h. During the reaction, a white solid was precipitated, filtered by suction, washed with absolute ethanol, and then recrystallized with absolute ethanol to obtain white needle-like crystals, namely intermediate 2, with a yield of 47.5% and a melting point of 235-236°C. FAB-MS m / z:352([M+H] + ); 1 H NMR (DMSO-d 6 )δ:10.15(s,1H,NH),7.78-7.59(m,3H,ArH),7.37(dd,J=5....
Embodiment 2
[0036] Repeat the method of embodiment 1, just carry out following modification:
[0037] 1. Modify the solvent dichloromethane used in step 1) to chloroform, and modify the reaction conditions to react at 30°C for 48h;
[0038] 2. Modify the solvent dichloromethane used in step 2) to chloroform, and modify the reaction conditions to react at 10°C for 12 hours;
[0039] 3. Modify the reaction conditions in step 3) to react at 50°C for 1 hour;
[0040] 4. Modify the solvent anhydrous tetrahydrofuran used in step 4.1) to anhydrous diethyl ether; modify the reaction conditions in step 4.2) to 200°C for 3 minutes, and at the same time, use 4 mL of nitrobenzene and The solvent consisting of 2mL xylene was modified to 6mL nitrobenzene.
[0041] The yield of the final product obtained in this implementation is 22%, and the melting point is 272-275°C, which is basically consistent with the literature value of 275-276°C. It is the same compound compared with the reference substance (...
Embodiment 3
[0043] Repeat the method of embodiment 1, just carry out following modification:
[0044] 1. Change the mixed solvent used for elution in step 3) to be composed of petroleum ether and ethyl acetate with a volume ratio of 10:1;
[0045] 2. Modify the reaction conditions in step 3) to react at 60°C for 1 hour;
[0046] 3. Modify the reaction conditions in step 4.2) to 150° C. for 15 minutes, and change the diethyl ether to tetrahydrofuran.
[0047] The yield of the final product obtained in this implementation is 20%, and the melting point is 272-275°C, which is basically consistent with the literature value of 275-276°C. It is the same compound compared with the reference substance (Shanghai Chunyou Biotechnology Co., Ltd.) by TLC method (expanded Agent: chloroform:methanol=9:1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com