A sequence that can be modified by 4' phosphopantethein and its method for immobilizing proteins
A technology of phosphopanthenylmercaptoethylamine and acyl carrier protein, which is applied in the field of immobilizing proteins, can solve the problems of unfavorable macromolecular substrate and product diffusion, limiting the wide application of immobilized proteins, and loss of protein (enzyme) activity, etc. Market value and application prospects, the effect of reducing inclusion body formation and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Cloning of an Escherichia coli acyl carrier protein (ACP) gene.
[0022] (1) Extraction of Escherichia coli total DNA
[0023] 1) Pick a single colony of Escherichia coli MG1655 in LB liquid medium and culture overnight at 37°C.
[0024] 2) Centrifuge at a centrifugal force of 5000g for 10 min, collect the bacteria, and obtain the genomic DNA of Escherichia coli by referring to the DNA extraction instructions of TIANGEN Company.
[0025] (2) Cloning of ACP gene
[0026] 1) Design 2 primers:
[0027]
[0028] 2) A fusion ACP gene (SEQ ID NO: 1) was obtained by amplifying with F1 and R1 primers, and the obtained fusion ACP gene fragment was digested with Nco I and EcoR I respectively, and connected to the pET28b vector.
[0029] (3) Fusion expression of ACP and green fluorescent protein (GFP)
[0030] 1) Construction of expression vector
[0031] The GFP gene was excised from the intermediate vector pET30a-GFP with EcoR I and Hind III, connected to the e...
Embodiment 2
[0034] Example 2 Cloning of a short peptide gene that can be modified by 4'-phosphopantethein and its fusion expression with the GFP gene.
[0035] (1) Cloning of short peptide genes that can be modified by 4'-phosphopantetheine
[0036] 1) Design 3 primers:
[0037]
[0038] 2) Using the GFP gene as a template, use primers F2, F3, and R2 to amplify to obtain a fusion gene S-GFP (the fusion gene S-GFP includes a gene that can be introduced by primers F2, F3, and R2 and can be introduced by 4' phosphopanthene Mercaptoethylamine-modified sequence (SEQ ID NO: 2). Primers R2, F3, and F2 were successively used as up primers in the first, second, and third rounds of PCR for PCR amplification.
[0039] The obtained fusion gene S-GFP fragment was digested with Hind III and EcoR I, and connected to pET28b vector.
[0040] (2) Expression of fused GFP gene (S-GFP)
[0041] 1) Construction of expression vector
[0042] The fused GFP gene (S-GFP) was digested with EcoR I and Hind III...
Embodiment 3
[0045] Example 3 Immobilization of the target protein GFP
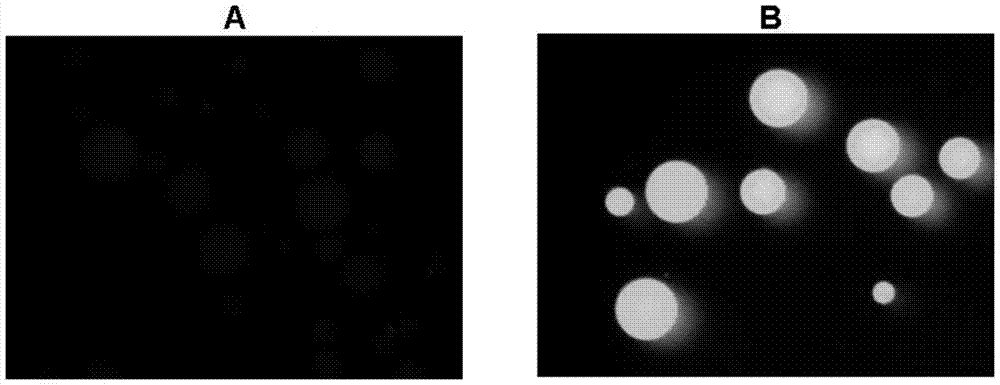
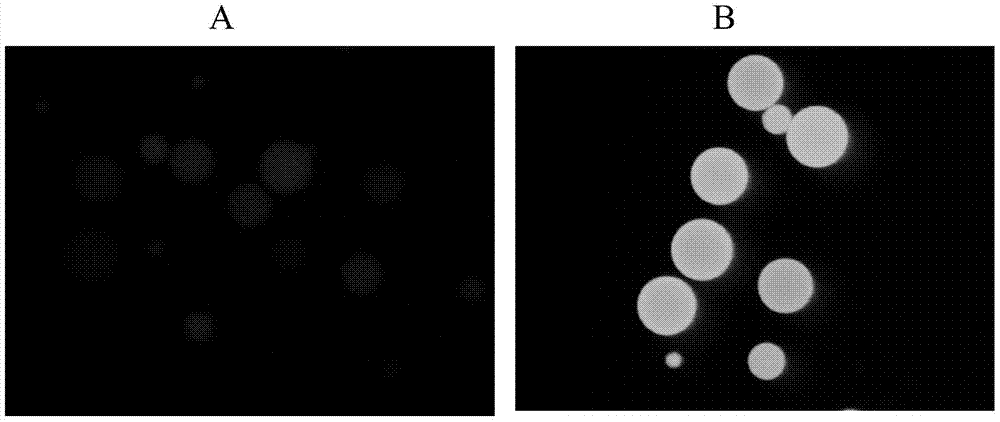
[0046] (1) Immobilization of fusion protein ACP-GFP
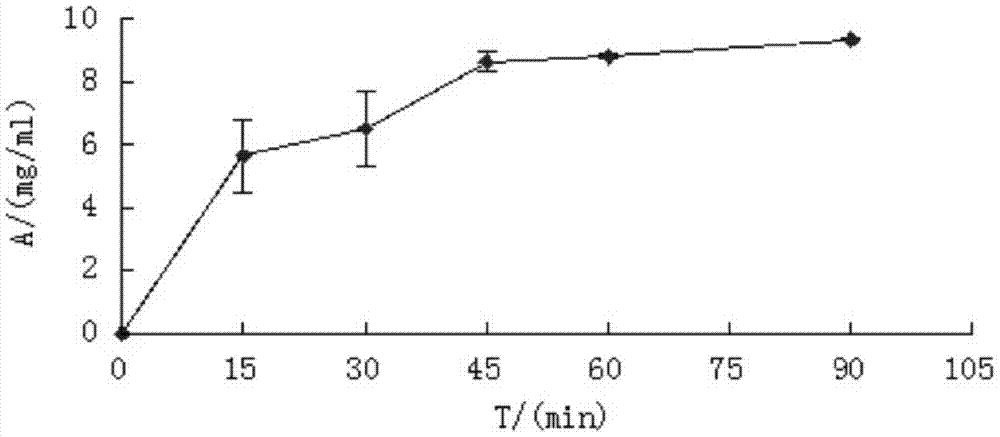
[0047] 10ml of Escherichia coli culture expressing the fusion protein ACP-GFP, the cells collected by centrifugation were ultrasonically disrupted in 4ml of buffer solution (50mM NaH2PO4, 0.3M NaCl, 20mM imidazole, pH 8.0), and purified by Ni column affinity chromatography , the obtained high-purity fusion protein was dialyzed, and the purified fusion protein was modified with 4' phosphopantethein with the enzyme AcpS, and then dialyzed into immobilization buffer (50mmol Tris HCl, 5mmol EDTA-Na, pH 8.0), To measure the protein concentration, mix about 1mg of recombinant protein with 0.2ml SulfoLink coupling resin (Thermo Scientific), incubate at 30°C for 1h, measure the change of recombinant protein concentration before and after immobilization, calculate the immobilized amount of recombinant protein, and use 50mML-cysteine· The column was washed 3 times with HCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com