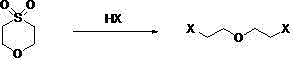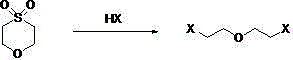Synthesis method of di(2-halogenated ethyl) ether
A technology of haloethyl and synthesis method, which is applied in the field of synthesis of fine chemical intermediates, can solve the problems of high toxicity of chlorohydrin, low reaction selectivity, and large pollution, and achieve slow reaction, good selectivity, and high reaction temperature suitable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: Preparation of raw material 1,4-thioxane-1,1-diox
[0014] According to the method of M.V.Rous (J.ORG.CHEM.2007, 72, 1143-47), 208 g of 1,4-thioxane, 300 ml of acetic acid and 400 ml of 30% hydrogen peroxide were stirred at room temperature for 2 days , after evaporating the solvent to dryness, recrystallized with dichloromethane-cyclohexane to obtain 204 g of 1,4-thioxane-1,1-dioxine, with a melting point of 76-78°C and a yield of 70%.
[0015] Product structure confirmation:
[0016] 1 HNMR (δppm, 400MHz , CDCl 3 ): 3.02-3.33(m, 4H); 4.05-4.23(m, 4H)
[0017] 13 CNMR (δppm, 100MHz, CDCl 3 ): 66.3; 53.0
[0018] IR: 2926, 1466, 1392, 1329, 1288, 1200, 1121, 1106
[0019] GC-MS: 15, 27, 28, 29, 43, 44(100%), 45, 65, 71, 72, 78, 109, 137
Embodiment 2
[0020] Embodiment 2: the preparation of two (2-chloroethyl) ethers
[0021] Add 68.1 grams of 1,4-thioxane-1,1-diox (0.5 moles) and 200 grams of hydrochloric acid (2 moles) with a mass concentration of 36.5% into a 500 mL four-neck flask, stir mechanically at 30°C and react in the gas phase Chromatography followed the reaction, and the reaction ended in 12 hours; layered, about 175 grams of the upper acid layer was set aside; the lower organic layer was washed with 50 ml of saturated sodium bicarbonate, layered, and the boiling range of 60-75 ° C was collected by water pump distillation to obtain Color liquid 59.2 grams, gas phase content 98.2%, yield 81.3%.
[0022] Product structure confirmation:
[0023] 1 HNMR (δppm, 400MHz , CDCl 3 ): 3.77 (4H, t, J =7.2 Hz, CH 2 O); 3.62 (4H, t, J = 7.2 Hz, CH 2 Cl)
[0024] 13 CNMR (δppm, 100MHz, CDCl 3 ): 71.3; 42.6
[0025] IR: 2866, 1301, 1126, 747, 659
[0026] GC-MS: 27, 28, 63, 65, 93, 95(100%), 142
Embodiment 3
[0027] Embodiment 3: apply mechanically hydrochloric acid to prepare two (2-chloroethyl) ethers
[0028] 68.1 grams of 1,4-thioxane-1,1-diox (0.5 moles), 175 grams of hydrochloric acid (about 1.5 moles) and 50 grams of new hydrochloric acid with a mass concentration of 36.5% (0.5 moles) were recovered in Example 2 ) into a 500mL four-neck flask, stirred mechanically and reacted at 30°C. The reaction was followed by gas chromatography, and the reaction was completed in 15 hours; the layers were separated, and the lower organic layer was washed with 50 mL of saturated sodium bicarbonate. Liquid 61.2 grams, gas phase content 98.5%, yield 84.3%. Product proton nuclear magnetic spectrum is identical with embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com