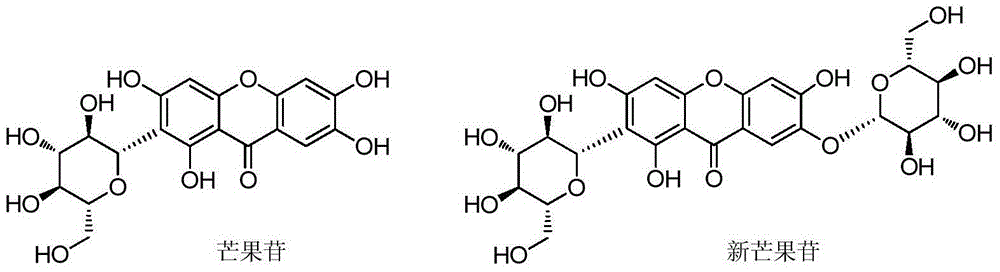
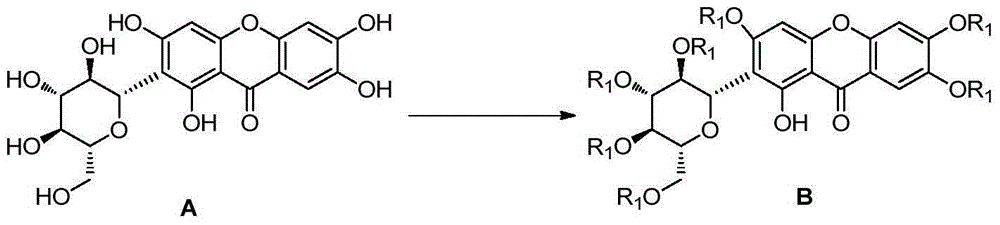
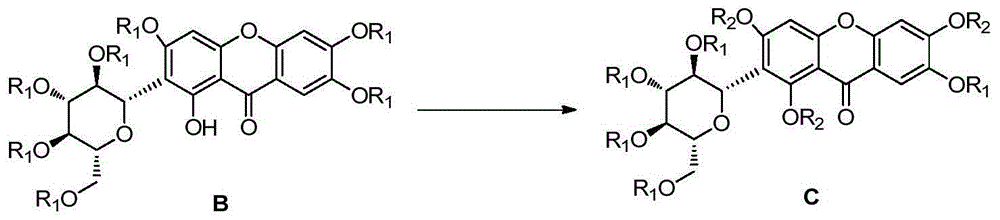
Mangiferin single-site derivative and preparation method and application thereof
A technology of mangiferin and derivatives, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of little difference in hydroxyl activity, difficulty in obtaining high-purity samples, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Example 1: 2-C-(2,3,4,6-O-tetraacetyl-β-D-glucosyl)-1-hydroxyl-3,6,7-triacetoxyxanthone synthesis
[0193] Take 5g (11.84mmol) of mangiferin and 8.16g (99.46mmol, 8.4eq) of sodium acetate and add it to 20ml of acetic acid, heat it to 120°C while stirring, add dropwise 10ml of acetic anhydride (105.79mmol, 8.9eq) to it, and reflux the reaction After 3h, 2ml of acetic anhydride (21.16mmol, 1.8eq) was added, and the reaction was continued for 0.5h. After the reaction, the reaction solution was poured into 200ml of ice water, stirred to separate out the solid, suction filtered, the filter cake was dissolved in dichloromethane, washed 3 times with saturated aqueous sodium bicarbonate solution, washed 3 times with water, washed 3 times with saturated aqueous sodium chloride solution. Dry over sodium sulfate, concentrate, and suck to dryness to obtain 7.94 g of a yellow solid, with a yield of 93.6%. Melting point 123.9-124.8°C, [α] D =-12.0° (c 1.00, CHCl 3 ).
[0194] 1...
Embodiment 2
[0195] Example 2: 2-C-(2,3,4,6-O-tetraacetyl-β-D-glucosyl)-1,3,6-tribenzyloxy-7-acetoxyxanthene Ketone synthesis
[0196]Take 5g (6.98mmol ) was dissolved in 100ml of acetone, potassium carbonate 11.57g (83.73mmol, 12eq), potassium iodide 1.16g (6.98mmol, 1eq), benzyl bromide 4.97ml (41.86mmol, 6eq) were added thereto, stirred at room temperature for 0.5h and then moved to Reflux reaction at 60°C for 13h. After the reaction was completed, the reaction solution was filtered, and the filtrate was concentrated. Petroleum ether: ethyl acetate = 1:1 column chromatography separation to obtain 5.35 g of white solid with a yield of 84.9%. Melting point 218.0-219.6°C, [α] D =-67.4° (c 0.95, CHCl 3 ).
[0197] 1 H NMR (400MHz, CDCl 3 )δ7.93(s,1H),7.68–7.52(m,4H),7.50–7.07(m,11H),6.83(s,1H),6.74(s,1H),6.09(t,J=9.7Hz ,1H),5.34–4.80(m,9H),4.20(dd,J=12.4,4.0Hz,1H),3.96(d,J=11.2Hz,1H),3.39(dt,1H),2.26(s, 3H), 2.01(s,3H), 1.96(s,3H), 1.89(s,3H), 1.77(s,3H). 13 CNMR (100MHz, CDCl 3...
Embodiment 3
[0198] Example 3: 2-C-(2,3,4,6-O-tetraacetyl-β-D-glucosyl)-7-hydroxyl-1,3,6-tribenzyloxyxanthone synthesis
[0199] Take 2-C-(2,3,4,6-O-tetraacetyl-β-D-glucosyl)-1,3,6-tribenzyloxy-7-acetoxyxanthone 5g ( 5.54mmol) was added to 100ml mixed solvent (methanol: acetone: water = 4:2:1), 3.41g (44.30mmol, 8eq) of ammonium acetate was added thereto, and refluxed at 60°C for 12h. After the reaction was completed, the reaction solution was concentrated and evaporated to dryness. The residue was dissolved in dichloromethane, washed three times with water and three times with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated. Dichloromethane: acetone = 30:1 column chromatography to obtain 4.02 g of yellow solid with a yield of 84.4%. Melting point 111.7-112.9°C, [α] D =-60.0° (c 1.00, CHCl 3 ).
[0200] 1 H NMR (400MHz, CDCl 3 )δ7.68–7.55(m,5H),7.49–7.22(m,11H),6.72(s,1H),6.67(s,1H),6.43(s,1H),6.08(t,J=9.7Hz ,1H),5.44–4.78(m,9H),4.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com