Methylene diphenyl amide compound and application thereof
A technology of methylene diphenylamides and compounds, which is applied in the field of methylene diphenylamides and their application as agricultural fungicides, and can solve the problem of unseen applications of methylene diphenylamides problems such as reports, to achieve the effect of high inhibitory activity, simple structure and good control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
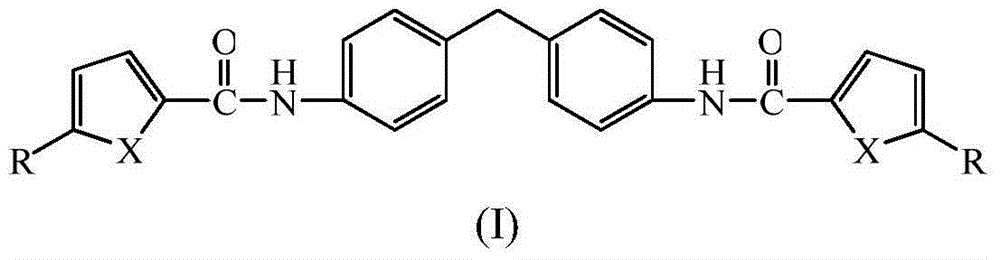
[0027] compound (C 23 h 18 N 2 o 4 ) preparation
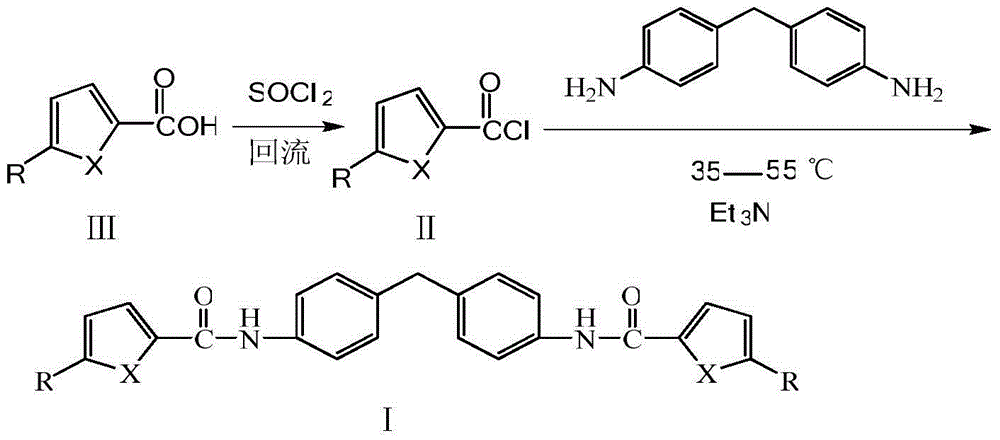
[0028] (1) Add 0.01mol of furan-2-carboxylic acid and 15mL of thionyl chloride to a 50mL three-necked flask, heat to 80°C and reflux for 2 hours, distill off excess thionyl chloride until no liquid flows out, Cool to room temperature to obtain furan-2-formyl chloride.
[0029] (2) Add 0.005mol 4,4,-diaminodiphenylmethane and 10mL dichloromethane into a 50mL three-neck flask, add 3mL triethylamine or pyridine, and add the newly prepared furan-2 dropwise under stirring at room temperature - formyl chloride. After the dropwise addition, continue the reaction at 35-55°C for 3 hours. After the reaction is complete, wash the reaction mixture with 10% hydrochloric acid, 10% NaOH and distilled water to neutrality, and place it in a refrigerator at 2-6°C until the solid precipitates Afterwards, filter, wash, and recrystallize with dimethyl sulfoxide and water to obtain a dark green crystalline product with a yield of 61%. The ...
Embodiment 2
[0033] compound (C 23 h 18 N 2 o 2 S 2 ) preparation
[0034] (1) Add 0.01mol thiophene-2-carboxylic acid and 15mL thionyl chloride to a 50mL three-neck flask, heat to 80°C and reflux for 2 hours, and distill off excess thionyl chloride until no liquid flows out, Cool to room temperature to obtain thiophene-2-formyl chloride.
[0035] (2) Add 0.005mol 4,4,-diaminodiphenylmethane and 10mL dichloromethane into a 50mL three-neck flask, add 3mL triethylamine or pyridine, and add the newly prepared thiophene-2 dropwise under stirring at room temperature - formyl chloride. After the dropwise addition, continue the reaction at 35-55°C for 3 hours. After the reaction is complete, wash the reaction mixture with 10% hydrochloric acid, 10% NaOH and distilled water to neutrality, and place it in a refrigerator at 2-6°C until the solid precipitates Afterwards, filter, wash, and recrystallize with dimethyl sulfoxide and water to obtain a light yellow crystal product with a yield ...
Embodiment 3
[0039] Determination of Antibacterial Activity of Methylene Diphenylamide Compounds against Phytopathogenic Fungi
[0040] 1. Plant pathogenic fungi tested
[0041] Rice sheath blight, wheat head blight, corn spot blight, rapeseed sclerotinia, tomato cinerea, potato infestation, melon anthracnose, grape white rot, citrus green mold, and apple ringworm .
[0042] 2. Experimental method
[0043] The test compound was dissolved in dimethyl sulfoxide, then added to tap water containing 0.1% Tween-80, and mixed uniformly to form a 200 mg / L test solution. This solution was added to the sterilized PDA medium, and at the same time, streptomycin at a concentration of 50 mg / L was added. Using the corresponding solution not containing the test compound as the blank control, make a drug-containing flat plate with uniform thickness for use, and repeat three times. Use a sterilized hole puncher to select Ф5mm well-grown, non-polluted, and uniformly growing bacterial cakes, and insert th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com