Preparation method of cefalonium
A technology of ceflonine and ceftiophene acid, which is applied in the field of preparation of ceflonine, can solve the problems of too many three wastes and cannot meet the requirements of industrialized production, and achieves the effects of less discharge of three wastes, being beneficial to the protection of production equipment, and fast drying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
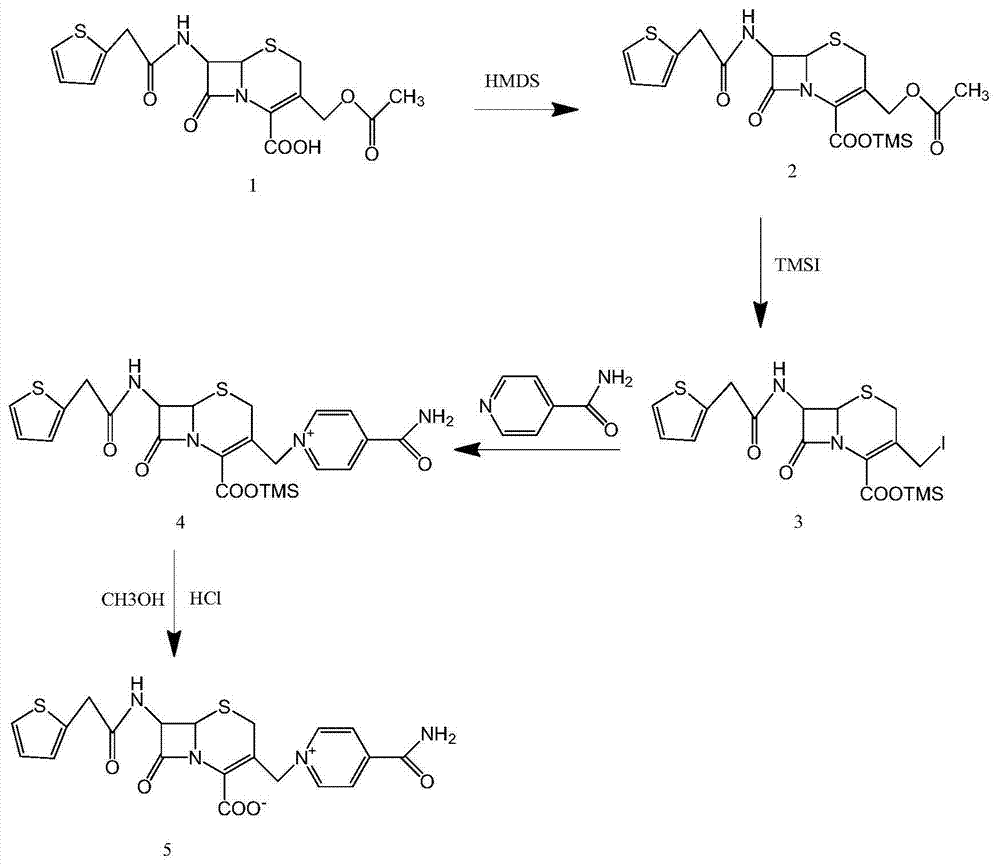
[0034] A preparation method of cefaroline, the steps are as follows:
[0035] (1) Add 43.7g cephalothin acid to 300ml dichloromethane, heat to reflux, add 1ml trimethylchlorosilane, 35ml hexamethyldisilazane, and continue the reflux reaction for 10 hours; cool down, add 30ml N,N -Diethylaniline, stirred for 30 minutes; added 33g iodotrimethylsilane, reacted at room temperature for 3 hours, detected 7-ACA residue, 7-ACA<0.5%, the reaction was over; cooled the system, added 27g isonicotinamide, 100ml di Chloromethane, reacted for 3 hours to obtain 4-amino-1-(8-oxo-7-(2-((2-thienyl)acetamido)-2-(trimethylsilyl)oxy)carbonyl )5-azabicyclo[4.2.0]thio-2-octen-3-yl)methyl)pyridinium salt (compound of formula 4);
[0036] (2) To the 4-amino-1-(8-oxo-7-(2-((2-thienyl)acetamido)-2-(trimethylsilyl)oxy group prepared in step (1) )carbonyl)5-azabicyclo[4.2.0]thio-2-octen-3-yl)methyl)pyridinium salt (compound of formula 4) was added dropwise methanol 35ml, and the mass concentration was 30...
Embodiment 2
[0039] A preparation method of cefaroline, the steps are as follows:
[0040] (1) Add 43.7g cephalothinic acid to 350ml dichloromethane, heat to reflux, add 1ml trimethylchlorosilane, 40ml hexamethyldisilazane, continue to reflux for 10 hours; cool down, add 28ml N,N -Diethylaniline, stirred for 30 minutes; added 35g iodotrimethylsilane, reacted at room temperature for 3 hours, detected 7-ACA residue, 7-ACA<0.5%, the reaction was over; cooled the system, added 24g isonicotinamide, 120ml di Chloromethane, reacted for 4 hours to obtain 4-amino-1-(8-oxo-7-(2-((2-thienyl)acetamido)-2-(trimethylsilyl)oxy)carbonyl )5-azabicyclo[4.2.0]thio-2-octen-3-yl)methyl)pyridinium salt (compound of formula 4);
[0041] (2) To the 4-amino-1-(8-oxo-7-(2-((2-thienyl)acetamido)-2-(trimethylsilyl)oxy group prepared in step (1) )carbonyl)5-azabicyclo[4.2.0]thio-2-octen-3-yl)methyl)pyridinium salt (Formula 4 compound) in the reaction system dropwise added methanol 34ml, added 26gFeCl 3 , 88ml of 6N...
Embodiment 3
[0044] A preparation method of cefaroline, the steps are as follows:
[0045] (1) Add 43.7g cephalothin acid to 290ml dichloromethane, heat to reflux, add 1ml trimethylchlorosilane, 38ml hexamethyldisilazane, continue to reflux for 10 hours; cool down, add 29ml N,N -Diethylaniline, stirred for 30 minutes; added 36g iodotrimethylsilane, reacted at room temperature for 3 hours, detected 7-ACA residue, 7-ACA<0.5%, the reaction was over; cooled the system, added 26g isonicotinamide, 100ml di Chloromethane, reacted for 3.5 hours to obtain 4-amino-1-(8-oxo-7-(2-((2-thienyl)acetamido)-2-(trimethylsilyl)oxy)carbonyl )5-azabicyclo[4.2.0]thio-2-octen-3-yl)methyl)pyridinium salt (compound of formula 4);
[0046] (2) To the 4-amino-1-(8-oxo-7-(2-((2-thienyl)acetamido)-2-(trimethylsilyl)oxy group prepared in step (1) ) carbonyl) 5-azabicyclo [4.2.0] thio-2-octen-3-yl) methyl) pyridinium salt (formula 4 compound) in the reaction system dropwise added methanol 33ml, adding 12ml mass concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com