Preparation method for regadenoson
A technology of regadeson and compound, which is applied in the field of preparation of regadeson, can solve the problems of unfavorable intermediate quality control and production scale-up, long reaction route, difficult reaction control, etc. The effect of processing operations and speeding up the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
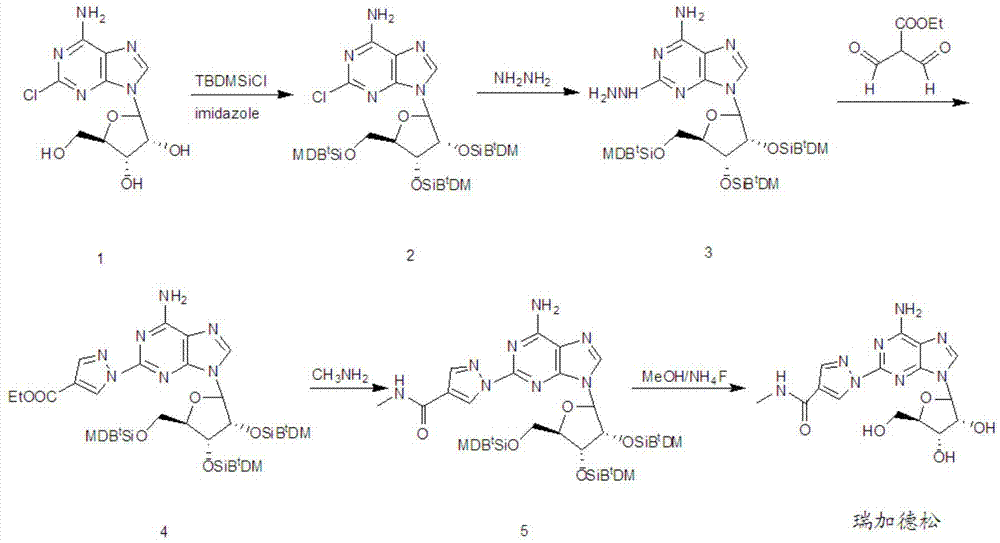
[0072] A preparation method for a compound shown in formula III, comprising:
[0073] Step a, in an organic solvent, under the action of a catalyst, using tert-butyldimethylsilyl chloride as a protective agent to protect the hydroxyl group of 2-chloroadenosine to obtain compound 2 shown in formula II;
[0074] Step b, compound 2 shown in formula II undergoes a substitution reaction with hydrazine hydrate in an alcoholic solvent to generate compound 3 shown in formula III;
[0075]
[0076] The preparation method of the compound shown in the formula III of the present invention firstly uses tert-butyldimethylsilyl chloride as a protective agent to protect the hydroxyl group of 2-chloroadenosine to obtain the compound 2 (2', 3' shown in the formula II ,5'-tri-tert-butyldimethylsilyl-2-chloroadenosine); then in an alcoholic solvent, compound 2 shown in formula II and hydrazine hydrate undergo a substitution reaction to generate compound 3 shown in formula III (2',3 ',5'-tri-t...
Embodiment 1
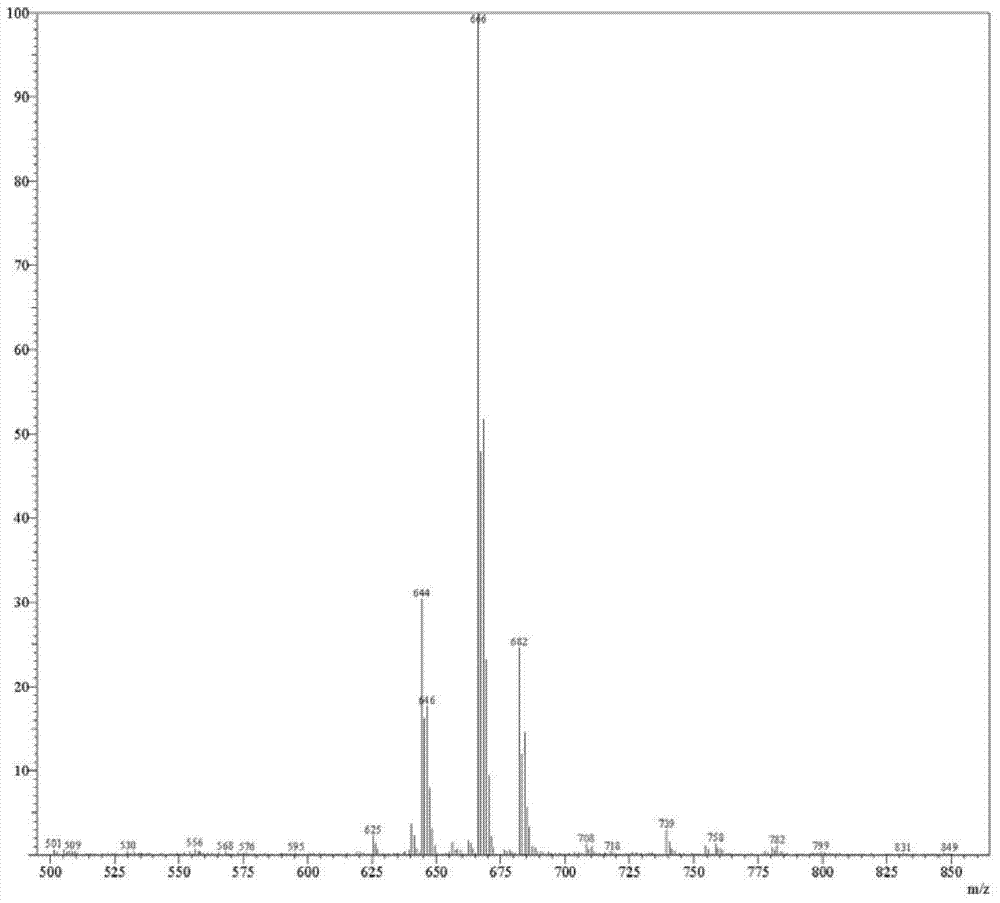
[0096] Example 1: Compound 2, the preparation of 2',3',5'-tri-tert-butyldimethylsilyloxy-2-chloroadenosine
[0097]
[0098] To 500 g of pyridine was added 50 g of compound 1. Continue to add 210g of tert-butyldimethylsilyl chloride and 90g of imidazole under stirring, stir and dissolve completely at 25°C, and react for 24 hours. TLC analysis and tracking, the raw materials are consumed, concentrated at 50 ° C, 100 Pa under reduced pressure to remove pyridine, added 650 g of dichloromethane to the solid to gradually dissolve, added 300 g of saturated sodium bicarbonate solution to the system, stirred for 30 minutes, there were a lot of bubbles After the bubbles disappeared, stand still for 30 minutes, separate the layers, and wash the dichloromethane phase once with 300 g of saturated sodium bicarbonate solution. The dichloromethane phase was washed three times with saturated sodium chloride solution, 400 g each time. The dichloromethane phase was dried with 100 g of anhy...
Embodiment 2
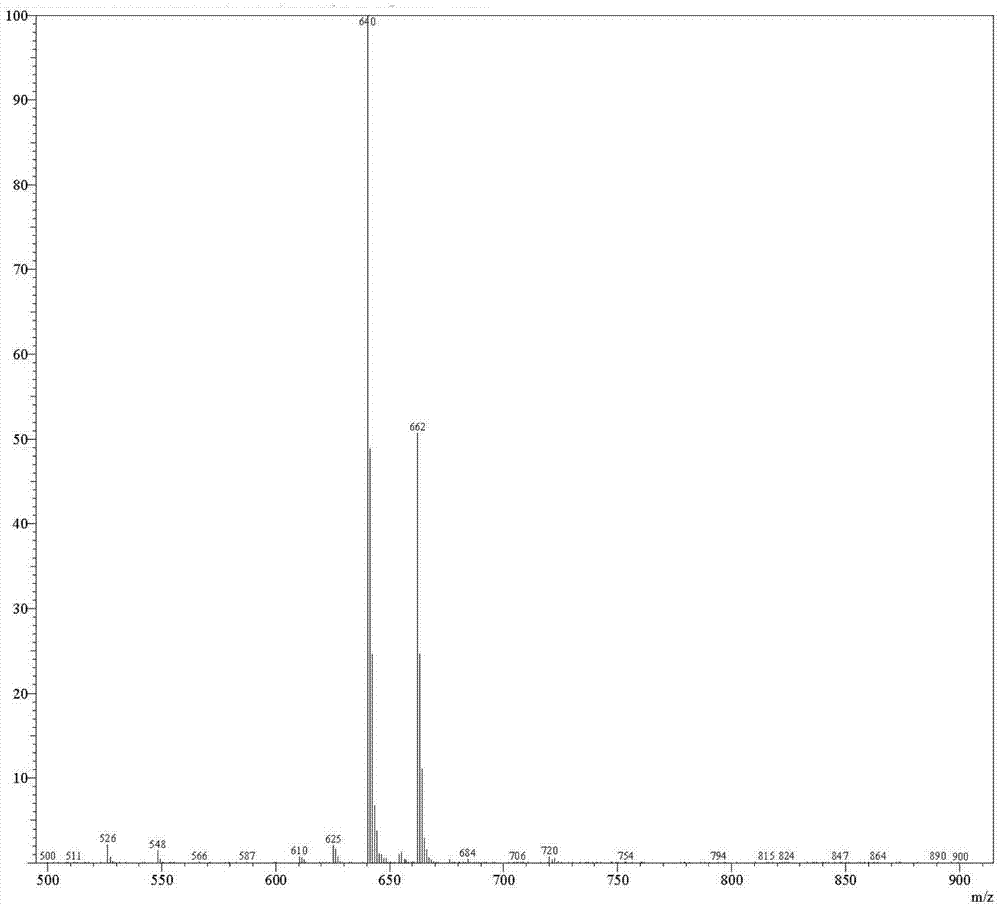
[0099] Example 2: Compound 3, the preparation of 2',3',5'-tri-tert-butyldimethylsilyloxy-2-hydrazinoadenosine
[0100]
[0101] 50 g of compound 2 was dissolved in 200 g of absolute ethanol, stirred and dissolved at 50°C. Under nitrogen protection, 80g of 80% hydrazine monohydrate was added, and the temperature rose to 70°C. After reacting for 20 hours, TLC followed the reaction process. After the raw materials were consumed, the reaction solution was slowly poured into 2 kg of ice water, stirred for 1 hour, and filtered. The filter cake was washed three times with purified water, each 100 g. Drain. Air-dried at 40°C for 12 hours to obtain compound 3. Yield 94%, purity 92%. Mass Spectrometry [M+Na] + =662.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com