Layered oxide material, preparation method, pole piece, secondary cell and application
A secondary battery and oxide technology, applied in the field of materials, can solve the problems of low electronic conductivity, difficult application, and low capacity, and achieve excellent cycle performance, low manufacturing cost, and good safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1 of the present invention provides a layered oxide material whose general chemical formula is: Na x Cu i Fe j mn k m y o 2+β ;
[0076] Among them, M is an element for doping and replacing the transition metal site, specifically Li + , Ni 2+ ,Mg 2+ ,Mn 2+ ,Zn 2+ ,Co 2+ , Ca 2+ , Ba 2+ ,Sr 2+ ,Mn 3+ ,Al 3+ ,B 3+ ,Cr 3+ ,Co 3+ ,V 3+ ,Zr 4+ , Ti 4+ ,Sn 4+ ,V 4+ ,Mo 4+ ,Mo 5+ , Ru 4+ , Nb 5+ ,Si 4+ ,Sb 5+ ,Nb 5+ ,Mo 6+ , Te 6+ one or more of
[0077] Said x, y, i, j, k, and β are respectively the molar percentages of corresponding elements; wherein the relationship between x, y, i, j, k, and β satisfies y+i+j+k=1, And x+my+2i+3j+4k=2(2+β); where 0.8≤x≤1; 0<i≤0.3; 0<j≤0.5; 0<k≤0.5; -0.02≤β≤0.02; m is the valence state of said M;
[0078] The space group of the layered oxide material is
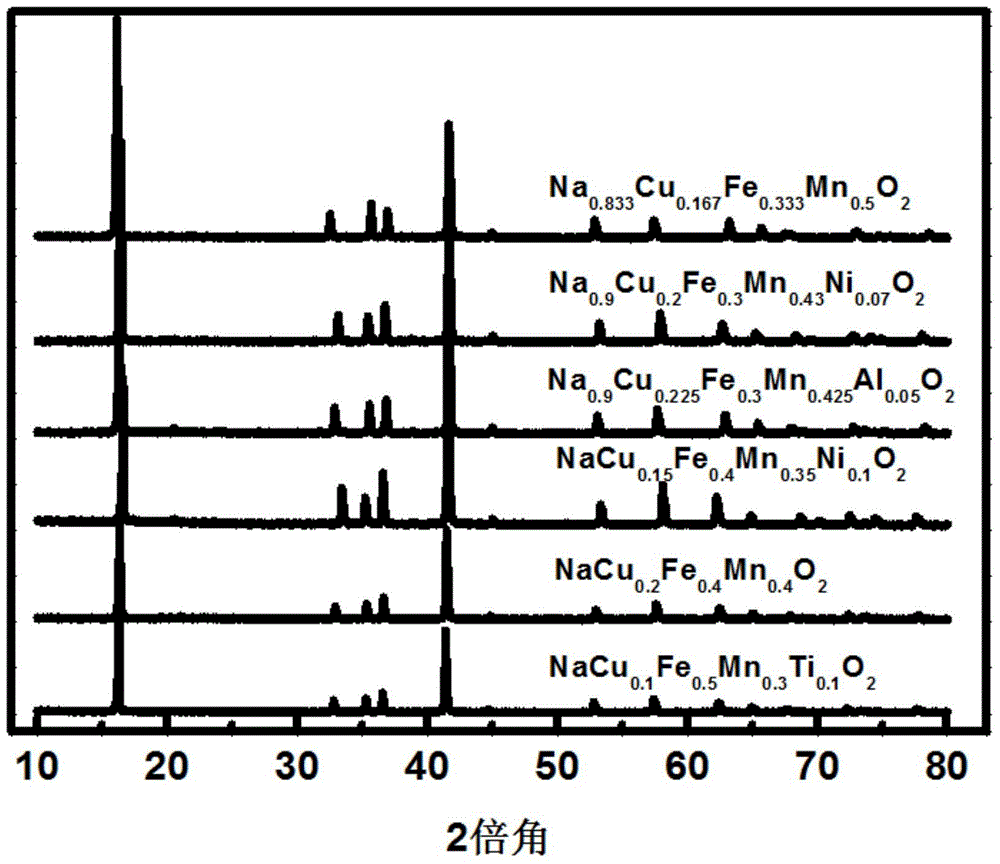
[0079] exist figure 1 The X-ray diffraction (X-ray diffraction, XRD) collection of a plurality of layered oxide materials of different elem...
Embodiment 2
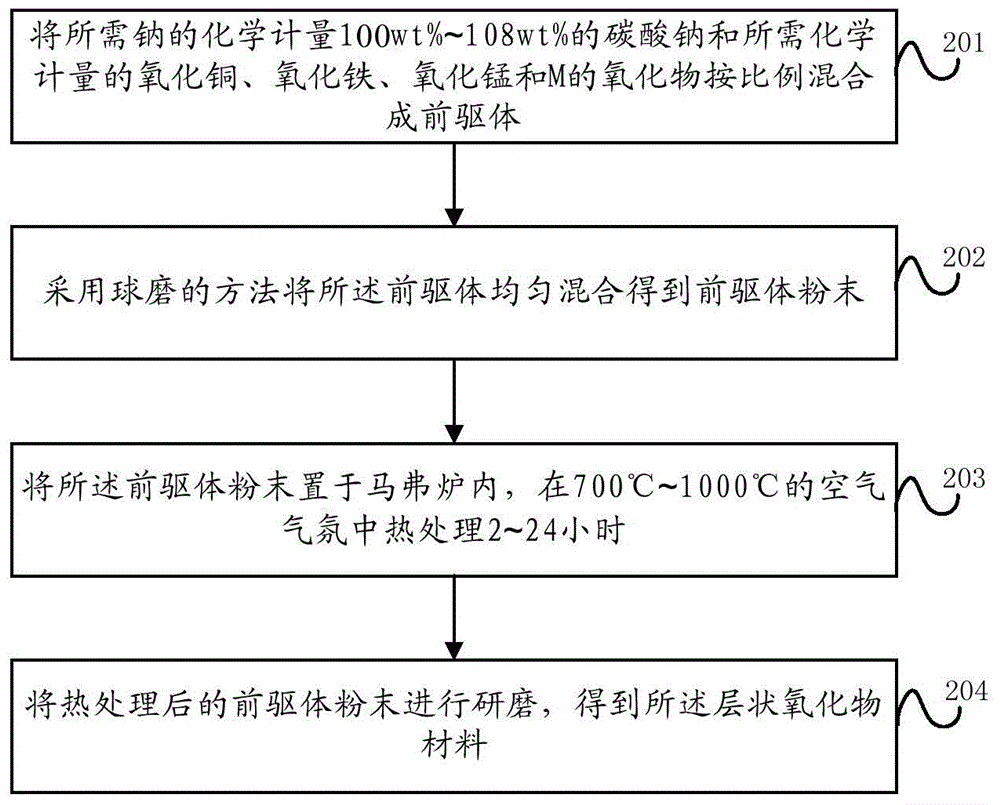
[0082] This embodiment provides a method for preparing a layered oxide material, specifically a solid phase method, such as figure 2 shown, including:
[0083] Step 201, mixing sodium carbonate with a required stoichiometric amount of 100wt% to 108wt% of sodium and required stoichiometric amounts of copper oxide, iron oxide, manganese oxide and M oxide in proportion to form a precursor;
[0084] Specifically, the M is specifically Li + , Ni 2+ ,Mg 2+ ,Mn 2+ ,Zn 2+ ,Co 2+ , Ca 2+ , Ba 2+ ,Sr 2+ ,Mn 3+ ,Al 3+ ,B 3+ ,Cr 3+ ,Co 3+ ,V 3+ ,Zr 4+ , Ti 4+ ,Sn 4+ ,V 4+ ,Mo 4+ ,Mo 5+ , Ru 4+ , Nb 5+ , Si 4+ ,Sb 5+ ,Nb 5+ ,Mo 6+ , Te 6+ one or more of.
[0085] Step 202, using a ball milling method to uniformly mix the precursor to obtain a precursor powder;
[0086] Step 203, placing the precursor powder in a muffle furnace, and heat-treating it in an air atmosphere at 700° C. to 1000° C. for 2 to 24 hours;
[0087] Step 204, grinding the heat-treated prec...
Embodiment 3
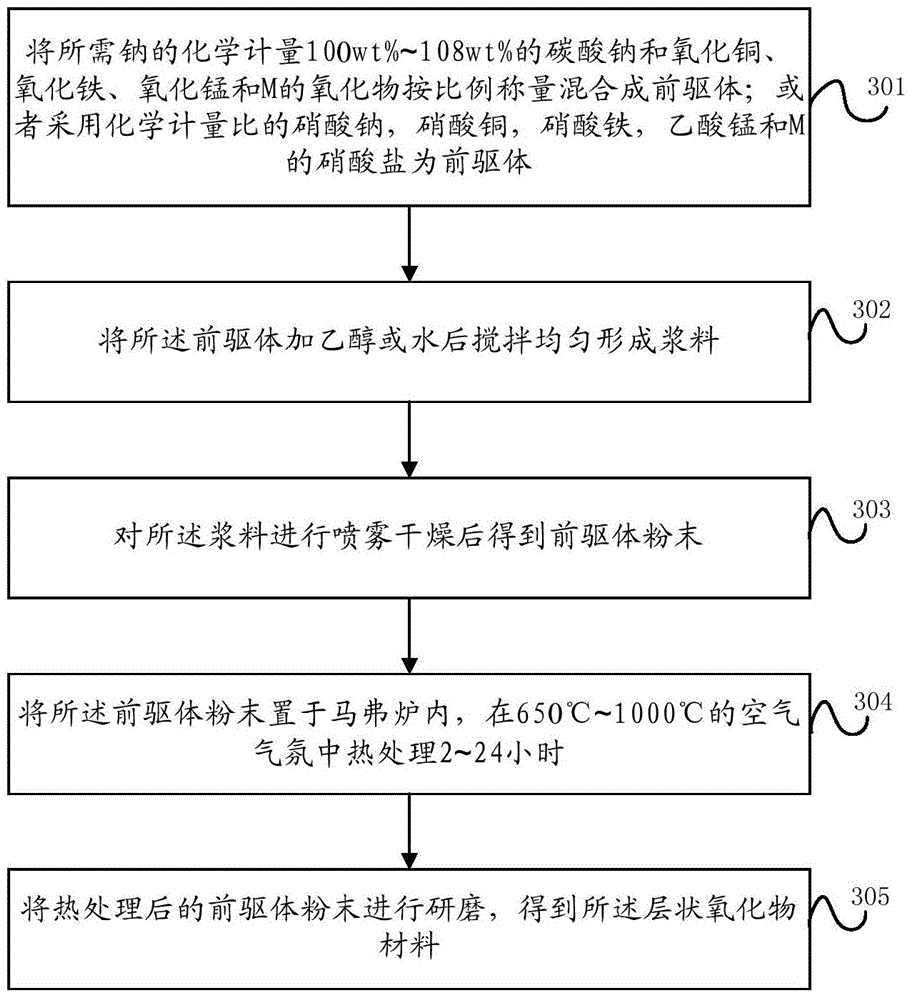
[0090] This embodiment provides a preparation method of a layered oxide material, specifically a spray drying method, such as image 3 shown, including:
[0091] Step 301, weighing and mixing sodium carbonate, copper oxide, iron oxide, manganese oxide and M oxide in proportion to the required sodium stoichiometric ratio of 100wt% to 108wt% to form a precursor; or using stoichiometric ratio of sodium nitrate, Copper nitrate, iron nitrate, manganese acetate and M nitrate as precursors;
[0092] Specifically, the M can be Li + , Ni 2+ ,Mg 2+ ,Mn 2+ ,Zn 2+ ,Co 2+ , Ca 2+ , Ba 2+ ,Sr 2+ ,Mn 3+ ,Al 3+ ,B 3+ ,Cr 3+ ,Co 3+ ,V 3+ ,Zr 4+ , Ti 4+ ,Sn 4+ ,V 4+ ,Mo 4+ ,Mo 5+ , Ru 4+ , Nb 5+ , Si 4+ ,Sb 5+ ,Nb 5+ ,Mo 6+ , Te 6+ one or more of.
[0093] Step 302, adding ethanol or water to the precursor and stirring evenly to form a slurry;
[0094] Step 303, spray-drying the slurry to obtain a precursor powder;
[0095] Step 304, placing the precursor powder i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com