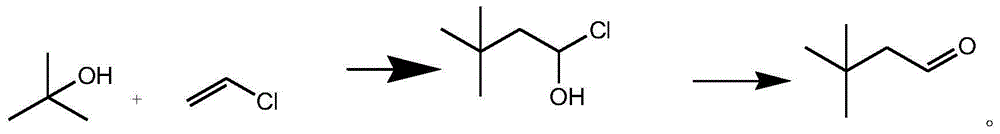
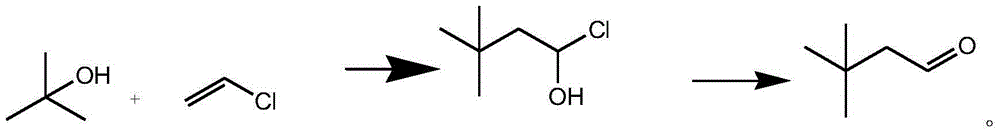
Preparation method for 3, 3-dimethylbutyraldehyde
A technology of dimethylbutyraldehyde and dimethylbutyl, which is applied in the new preparation field, can solve the problems of high industrial cost, high reaction energy consumption, and high cost, and achieve high production safety, mild reaction conditions, and production process short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
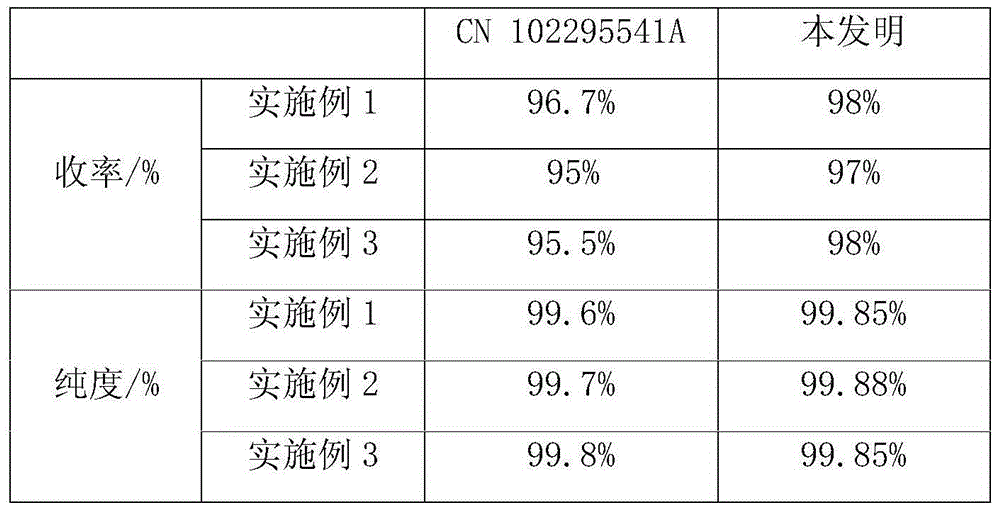
Embodiment 1
[0022] The preparation method of 3,3-dimethylbutanal of the present invention, it specifically comprises the following steps:
[0023] Step 1. Put 100 kg of dichloromethane into the reaction kettle, turn on the stirring and cooling cycle, and when the temperature drops to -10°C--5°C, add 2.5 kg of ferric chloride, and add tert-butanol dropwise within 5 hours 60 kg, and then 45 kg of vinyl chloride gas was introduced within 3 hours, and after the ventilation was completed, it was kept at -5°C--2°C for 3 hours;
[0024] Step 2. After the reaction is over, pour 30 kg of deionized water into the reaction kettle, stir well, separate the organic layer, add 12 kg of sodium sulfate to dry, then concentrate and recover dichloromethane at 30°C-45°C until exhausted. Continue vacuum distillation, collect 60°C-64°C / 20mmHg fractions, and obtain 110.2 kg of colorless liquid 1-hydroxy-3,3-dimethylbutyl chloride;
[0025] Step 3. Put 100.2 kg of 1-hydroxy-3,3-dimethylbutyl chloride into the r...
Embodiment 2
[0027] The preparation method of 3,3-dimethylbutanal of the present invention, it specifically comprises the following steps:
[0028] Step 1. Put 200 kg of dichloromethane into the reaction kettle, turn on the stirring and cooling cycle, and when the temperature drops to -10°C--5°C, add 6 kg of aluminum trichloride, and add tert-butanol dropwise within 5 hours 122 kg, and then 105 kg of vinyl chloride gas was introduced within 3 hours, and after the ventilation was completed, it was kept at -5°C--2°C for 3 hours;
[0029] Step 2. After the reaction is over, pour 70 kg of deionized water into the reaction kettle, stir well, separate the organic layer, add 30 kg of sodium sulfate to dry, and concentrate and recover dichloromethane at 30°C-45°C until exhausted. Continue vacuum distillation, collect fractions at 60°C-64°C / 20mmHg, and obtain 224.2 kg of colorless liquid 1-hydroxy-3,3-dimethylbutyl chloride;
[0030] Step 3. Put 205.6 kg of 1-hydroxy-3,3-dimethylbutyl chloride int...
Embodiment 3
[0032] The preparation method of 3,3-dimethylbutanal of the present invention, it specifically comprises the following steps:
[0033] Step 1. Put 320 kg of dichloromethane into the reaction kettle, turn on the stirring and cooling cycle, and when the temperature drops to -10°C--5°C, add 6 kg of methanesulfonic acid, and dropwise add 200 kg of tert-butanol within 5 hours kg, and then 150 kg of vinyl chloride gas was introduced within 3 hours, and after the ventilation was completed, it was kept at -5°C--2°C for 3 hours;
[0034] Step 2. After the reaction is over, put 100 kg of deionized water into the reaction kettle, stir well, separate the organic layer, add 16 kg of sodium sulfate to dry, concentrate and recover dichloromethane at 30°C-45°C until exhausted, Continue vacuum distillation, collect fractions at 60°C-64°C / 20mmHg, and obtain 366.6 kg of colorless liquid 1-hydroxy-3,3-dimethylbutyl chloride;
[0035] Step 3. Put 326.6 kg of 1-hydroxy-3,3-dimethylbutyl chloride i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com