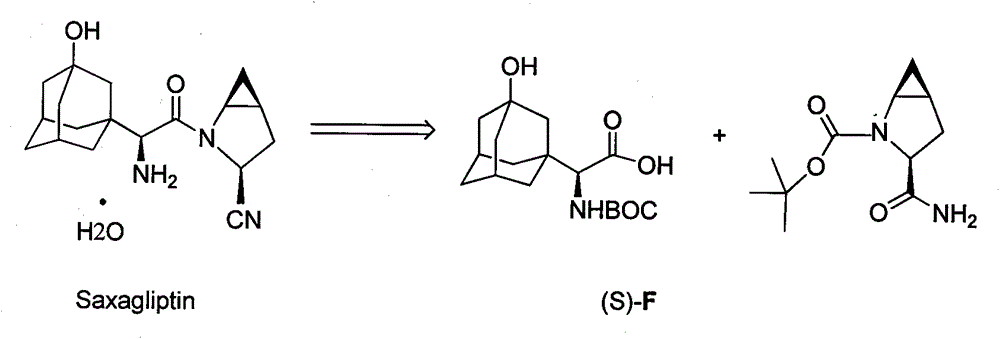
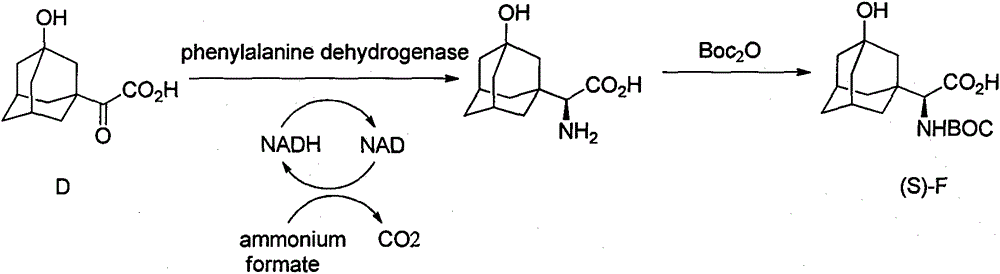
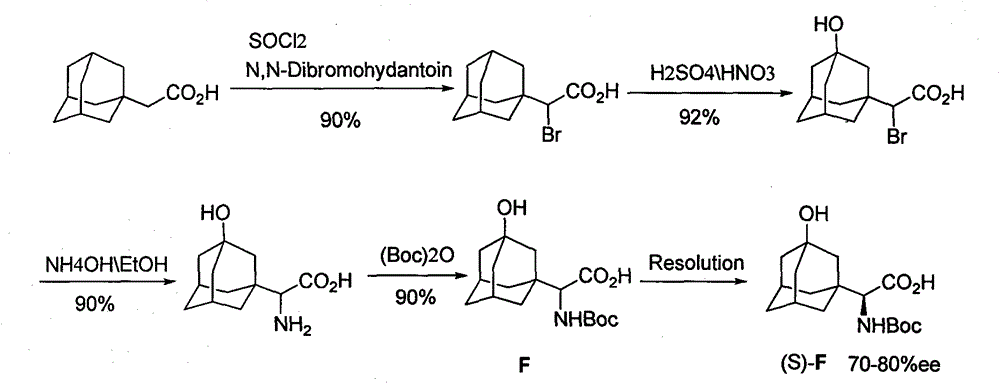
Preparation method for unnatural amino acids
A technology of unnatural amino acid and adamantyl glycine, which is applied in the chemical pharmaceutical field of pharmaceutical intermediates, can solve problems such as unfavorable safety and environmental protection, complicated reagent application, and increased production costs, achieving excellent quality, short routes, and reduced production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of 3-hydroxy-1-adamantanecarboxylic acid (B)
[0058] Add 10g (0.056mol) adamantanecarboxylic acid (A), 2.5gKOH (0.045mol), 126ml water, 2ml tert-butanol into a 250ml three-necked flask, heat to 45°C, add 10.6g (0.067mol) in batches within half an hour mol) potassium permanganate, after the addition, the temperature was raised to 60° C. and kept at this temperature for eight hours, and 20 ml of 10% sodium sulfite solution was added to quench unreacted potassium permanganate. Filtrate while hot to obtain a white clear liquid, cool the filtrate to room temperature, adjust to pH = 1 with concentrated hydrochloric acid, obtain a white turbid liquid, stir overnight, filter to obtain a white powdery solid 3-hydroxy-1-adamantanecarboxylic acid (B) 9.36 g, yield 86%.
Embodiment 2
[0060] Preparation of 3-Hydroxy-1-adamantanylmethylketone (C)
[0061] Add 15g (0.076mol) compound (B) and 30ml thionyl chloride to a 100ml round bottom flask, heat and reflux for 6 hours, remove excess thionyl chloride by distillation under reduced pressure, cool to obtain a white solid, add 50ml petroleum ether Dissolve and set aside.
[0062] Add 3.0g (0.130mol) sodium metal and 100mL petroleum ether into a 500mL three-necked flask, and slowly add a mixed solution of 30ml (0.192mol) diethyl malonate and 50mL petroleum ether dropwise at room temperature until the sodium metal reacts completely Afterwards, the above-mentioned 50 ml petroleum ether solution in which adamantanecarbonyl chloride was dissolved was added dropwise, and stirred overnight at room temperature. Add 200ml of distilled water, separate the organic layer, concentrate the solvent to obtain a light yellow oil, add 6mL of concentrated sulfuric acid, 18mL of distilled water, 60mL of glacial acetic acid, stir ...
Embodiment 3
[0064] Preparation of 2-(3-hydroxy-1-adamantyl)glyoxylic acid (D)
[0065] Add 3.0g (0.015mol) compound C, 100ml 2% KOH solution, 12ml tert-butanol in a 250ml three-necked flask, heat to 40°C, add 4.3g (0.027mol) potassium permanganate in batches within 1 hour, keep After reacting at 40°C for 4 hours, 20 mL of 10% sodium sulfite solution was added to quench unreacted potassium permanganate. Suction filtration while hot, and the filter cake was washed with 10ml hot water. The filtrate was cooled to room temperature, adjusted to pH = 1 with concentrated hydrochloric acid, extracted three times with ethyl acetate, the combined organic layers were washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure to an oil, and washed with ethyl acetate:petroleum ether ( 1:1) to obtain 3.12 g of white solid, yield 90%. mp 162-163°C; 1 H-NMR (400 MHz, DMSO-d6) 6: 3.32 (br s, 1H), 2.16 (s, 2H), 1.74-1.42 (m, 12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com