Liquid-chip kit for quantitatively detecting concentration of lipoprotein phospholipase A2 in sample and preparation method thereof
A quantitative detection and lipoprotein technology, applied in measurement devices, instruments, disease diagnosis, etc., can solve problems such as high false negative rate, and achieve the effects of high accuracy, high speed, and wide linear range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
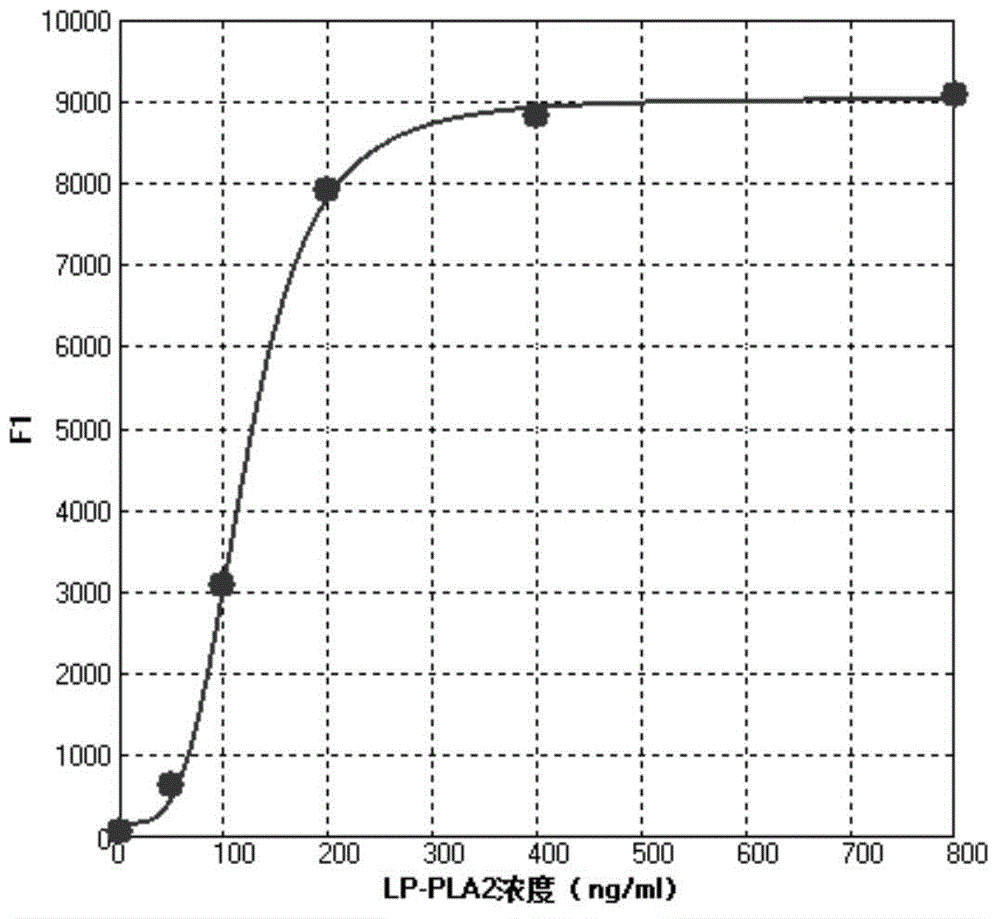
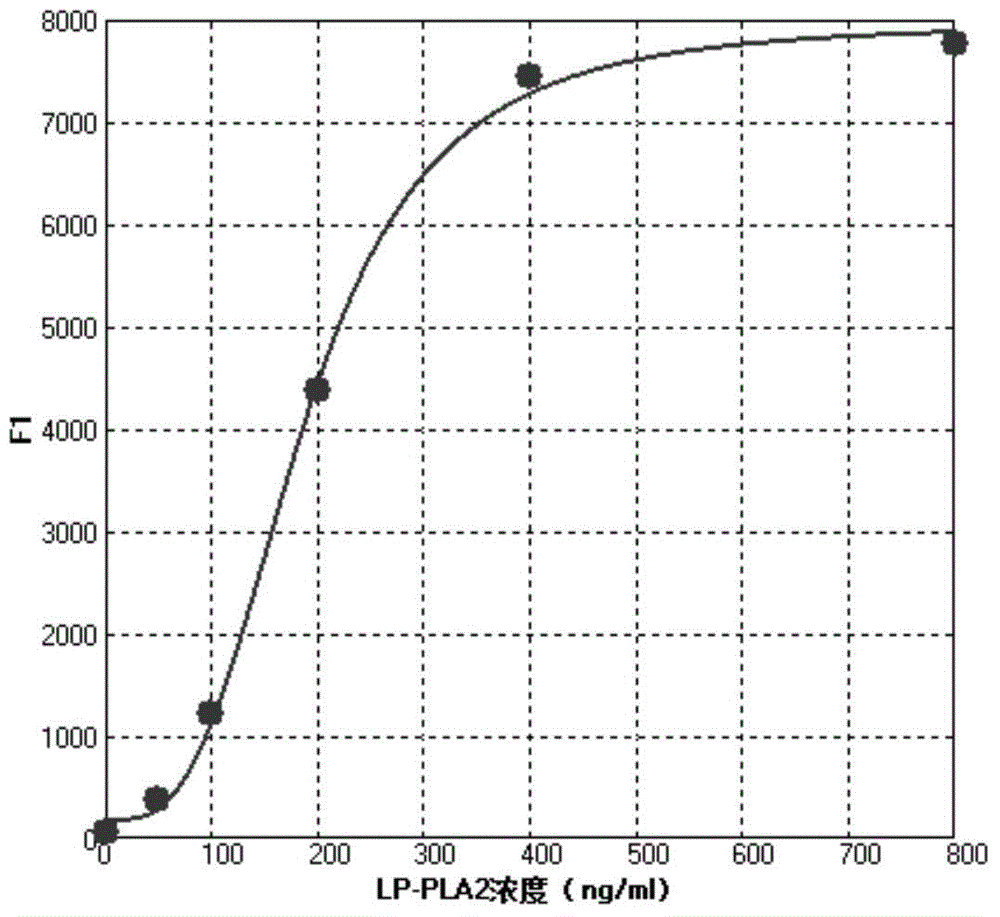
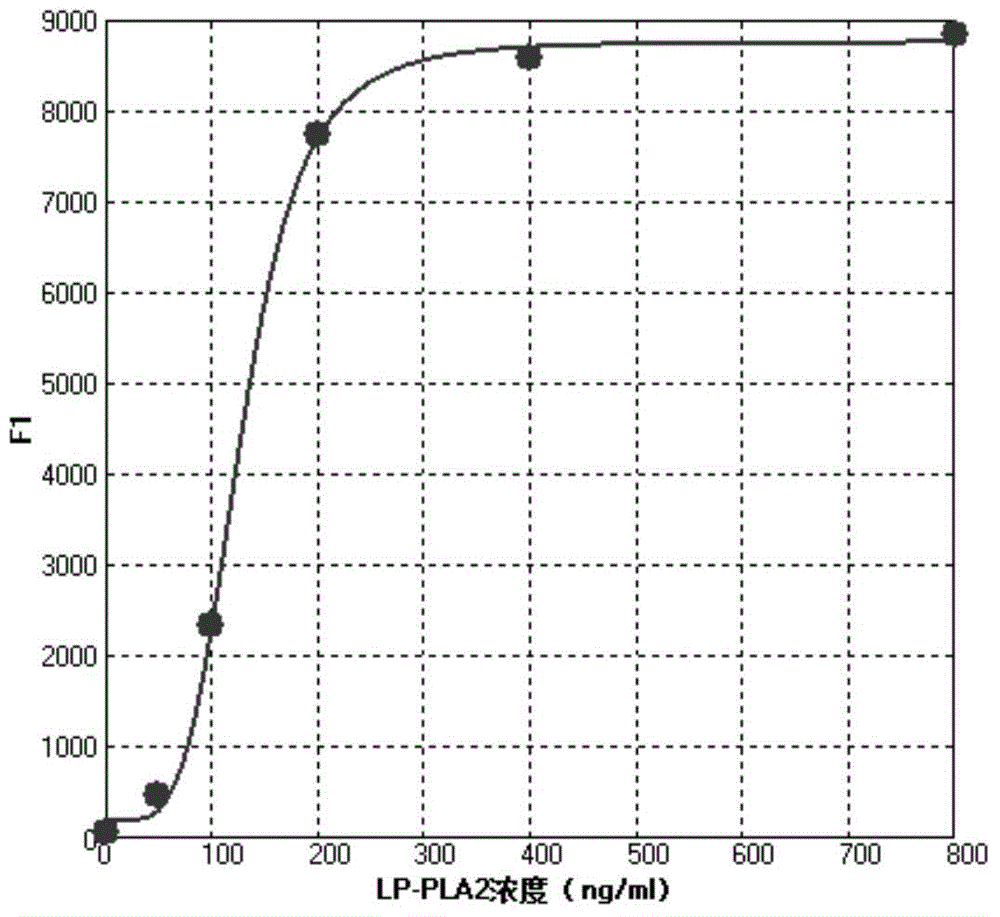
[0035] The operation method of the lipoprotein phospholipase A2 (LP-PLA2) concentration liquid chip detection kit of the present invention is as follows:
[0036] 1. Reconstitute the standard and quality control with deionized water and mix well, add standard 50 μl / well, quality control and sample 10 μl and 40 μl sample buffer / well to the microplate respectively;
[0037] 2. Dilute the antibody-coated magnetic particles by 25 times, then add 50 μl of magnetic particles to the microwell plate, and shake at room temperature for 120 minutes;
[0038] 3. Wash the plate twice with a plate washer;
[0039] 4. Add 25 μl / well of biotin-labeled monoclonal antibody to the microwell plate, and place it on a shaker for 60 minutes at room temperature;
[0040]5. Add 25 μl / well of streptavidinized phycoerythrin to the microwell plate, and place it on a shaker for 30 minutes at room temperature;
[0041] 6. Wash the plate twice with a plate washer;
[0042] 7. Add 150 μl / well sheath solut...
Embodiment 2
[0046] Prepare the streptavidinized phycoerythrin for this kit by:
[0047] Streptavidinylation of Phycoerythrin:
[0048] 1) Desalination treatment of phycoerythrin
[0049] Purified R-PE is generally stored in 60% saturated ammonium sulfate buffer solution, and the supernatant must be removed by centrifugation before use, and resuspended in 50mmol / L PBS, and ammonium sulfate is removed using a desalting column.
[0050] 2) Derivatization of phycoerythrin
[0051] Dissolve 4.5 mg of phycoerythrin in 1.0 mL of phosphate buffer, add 10 μL of 3-(2-pyridyldimercapto)propionic acid N-hydroxysuccinimide ester (SPDP) anhydrous methanol solution (4 mg / mL), Make the molar ratio of SPDP to phycoerythrin about 10, react at room temperature for 120 minutes, desalt through Sephadex gel chromatography, equilibrate and elute with phosphate buffer, and collect the peak of phycoerythrin solution.
[0052] 3) Thioylation of streptavidin
[0053] Get 0.5 mg of streptavidin, add the above-me...
Embodiment 3
[0059] Prepare the kit by:
[0060] Preparation of microspheres coated with monoclonal antibodies against lipoprotein phospholipase A2: Take 5.0×10 microspheres without coating 6 One, add 60 μL of 100 mM sodium dihydrogen phosphate buffer solution with a pH value of 6.2, 20 μL of 25 mg / mL N-hydroxysulfosuccinimide solution and 20 μL of 25 mg / mL carbodiimide solution for activation, and then add specific 25 μg of specific monoclonal antibody for coating; then add blocking solution containing 1% bovine serum albumin for blocking; finally store in 10mM phosphate buffer saline in the dark at 4°C;
[0061] Preparation of concentration gradient standard and quality control of lipoprotein phospholipase A2: 10 mM sodium phosphate buffer containing 1% mass fraction of newborn bovine serum, 0.01% Proclin300, 1% bovine serum albumin, 0.05% Triton X-100 Solution (PH7.0) The pure product of the marker was prepared into concentration gradient standards with marked concentrations of 0, 50, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com