Method for cyclic utilization of chlorine gas and coproduction of manganese chloride in chloride pyridine production process
A technology for the production process of pyridinium chloride, which is applied in the production fields related to pyridinium chloride, can solve the problems of strong corrosiveness of hydrochloric acid, high storage and transportation costs, restrictions on the development of hydrochloric acid electrolysis industry, and incomplete conversion of hydrogen chloride, etc., and achieve good environmental protection benefits and Economic benefits, equipment requirements and operating costs are small, and the effect of recycling is realized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
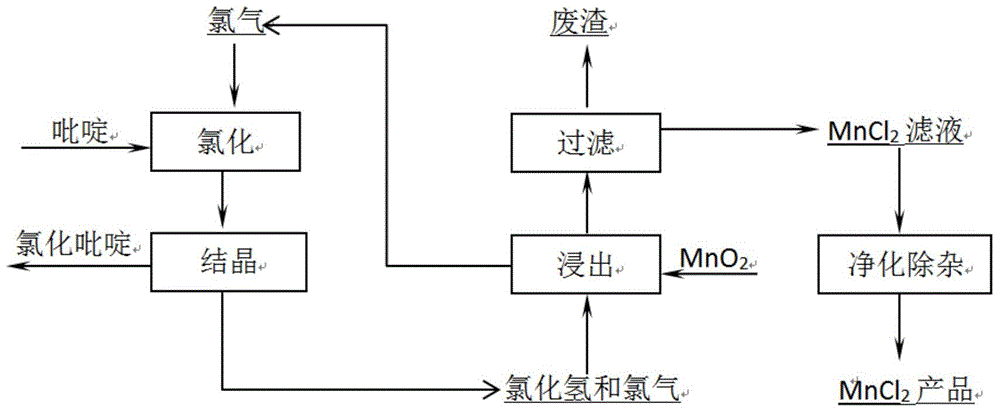
[0037] Catalyst (20% CoCl 2 , 80% LaCl 3 ) 400g, add pyridine in the vaporizer, the temperature of the vaporizer is controlled at 200°C, feed nitrogen into the vaporizer to saturate the pyridine, the nitrogen flow rate is 7L / h, and the pyridine content is 3% at this time, feed chlorine gas, and the chlorine flow rate is 2.24L / h , n Cl2 :n pyridine =8:1, the temperature of the reaction tube was raised to 300°C, and the content of solid components in the crystallizer was analyzed. In the solid content, 2,3,5,6-tetrachloropyridine is 5.6%, pentachloropyridine is 84.5%, and there are a small amount of dichloropyridine and trichloropyridine. The gas from the crystallizer is passed into the slurry tank for leaching. The mixed gas contains 56.34% hydrogen chloride and the rest is mainly chlorine.
Embodiment 2
[0039] Pyridine chlorination process is identical with embodiment 1. The leaching process operates as follows:
[0040] The manganese oxide ore sample was taken from a manganese mine in Xiangxi, Hunan. The main manganese mineral is pyrolusite. The chemical composition analysis is shown in Table 1. The operation steps are as follows:
[0041] (1) Cross 100 mesh sieves after manganese oxide ore is crushed;
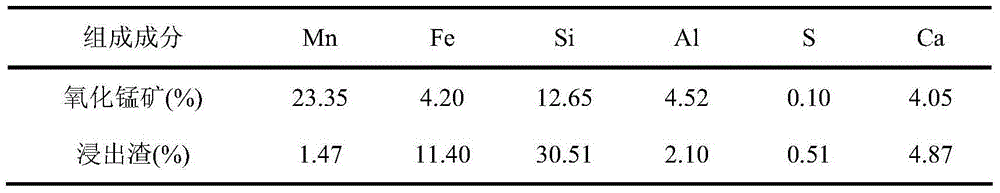
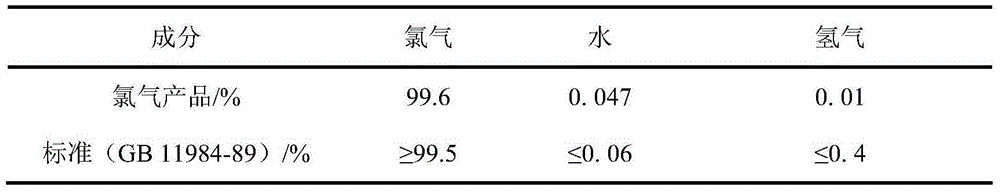
[0042] (2) Pass the mixed gas from the crystallizer into the above-mentioned manganese oxide slurry pool, the temperature of the slurry pool is 30°C, the rotation speed is 300r / min, add 100ml of water, 20g of manganese oxide, and leach for 10 hours. The leaching rate of manganese was 95.8%, and the chemical composition of the leaching residue was analyzed, the results are shown in Table 1 respectively. The chemical composition of the chlorine gas collected during the leaching process was analyzed. The results are shown in Table 2 respectively.
[0043] Table 1: Analysis...
Embodiment 3
[0051] Pyridine chlorination process is identical with embodiment 1. The leaching process operates as follows:
[0052] The manganese oxide ore sample was taken from a manganese mine in Yongzhou, Hunan (manganese grade 18.65%). The main manganese mineral is pyrolusite, and its chemical composition analysis is shown in Table 4. The operation steps are as follows:
[0053] (1) Pass through a 100-mesh sieve after the manganese oxide ore is crushed;
[0054] (2) Pass the mixed gas from the crystallizer into the above-mentioned manganese oxide slurry pool, the temperature of the slurry pool is 30°C, the rotation speed is 300r / min, add 100ml of water, 25g of manganese oxide, and leach for 10 hours. The leaching rate of manganese was 93.6%, and the chemical composition of the leaching residue was analyzed, the results are shown in Table 4 respectively. The obtained gas product chlorine has a quality of 99.08%.
[0055] Table 4: Analysis of chemical composition of ore samples
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com