Leave-on near-infrared lysosome probe
A lysosome and near-infrared technology, used in the synthesis and application of fluorescent probes, can solve the problems of fluorophore quenching, high cellular phototoxicity, and susceptibility to biological autoluminescence interference, and achieve low cytotoxicity and light stability good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of malachite green-based probe targeting lysosome
[0034] Compound b: Dissolve 6.1g of compound a, 9.8g of butyl bromobutyrate and 15g of potassium carbonate in 250ml of acetone, stir at room temperature for 12 hours, filter out potassium carbonate after the reaction, spin the filtrate to dryness, dissolve in 500ml of ethyl acetate, Washed with water (150ml×3), dried over anhydrous sodium sulfate, and spin-dried to obtain 10.6g white solid b, yield 89.4% Characterization data: 1H NMR (CDCl3, 400MHz): δ=9.88(s, 1H), 7.68(d, J=6.0Hz, 2H), 6.96(d, J=5.6Hz, 2H), 4.22(q, J=6.8Hz, 2H), 3.95(t, J=5.6Hz, 2H), 2.31(t, J= 7.6Hz, 2H), 2.12(m, J=7.2Hz, 2H), 1.31(t, J=4.8Hz, 3H); 13CNMR(CDCl3, 100MHz):
[0035] 190.1, 172.0, 165.2, 130.4, 114.3, 71.3, 59.6, 30.1, 25.4, 13.7
[0036]Compound d: Dissolve 2g of compound b, 2.57g of compound c and 6g of anhydrous zinc chloride in 120ml of absolute ethanol, heat to reflux for 24 hours under the protection of argon, spin dry ...
Embodiment 2
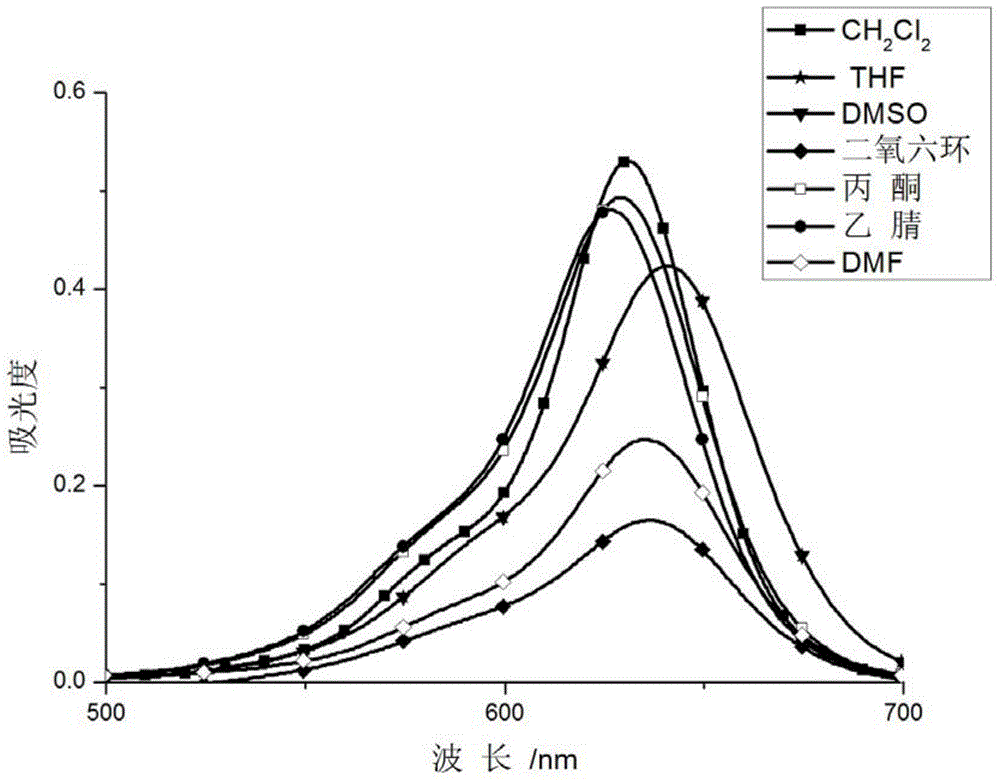
[0040] Prepare 10uM probe solutions in different solvents (dichloromethane, methanol, tetrahydrofuran, dimethyl sulfoxide, dioxane, acetone, acetonitrile, N,N-dimethylformamide) for UV-visible spectrophotometer and Fluorescence spectrophotometer test.
[0041] The results showed that the absorption of the probe shifted slightly at 620nm in different solvents, but the fluctuation was not large ( figure 1 ); there is no fluorescence in different solvents, and the ultraviolet absorption and fluorescence emission of the probe do not change with the change of polarity.
Embodiment 3
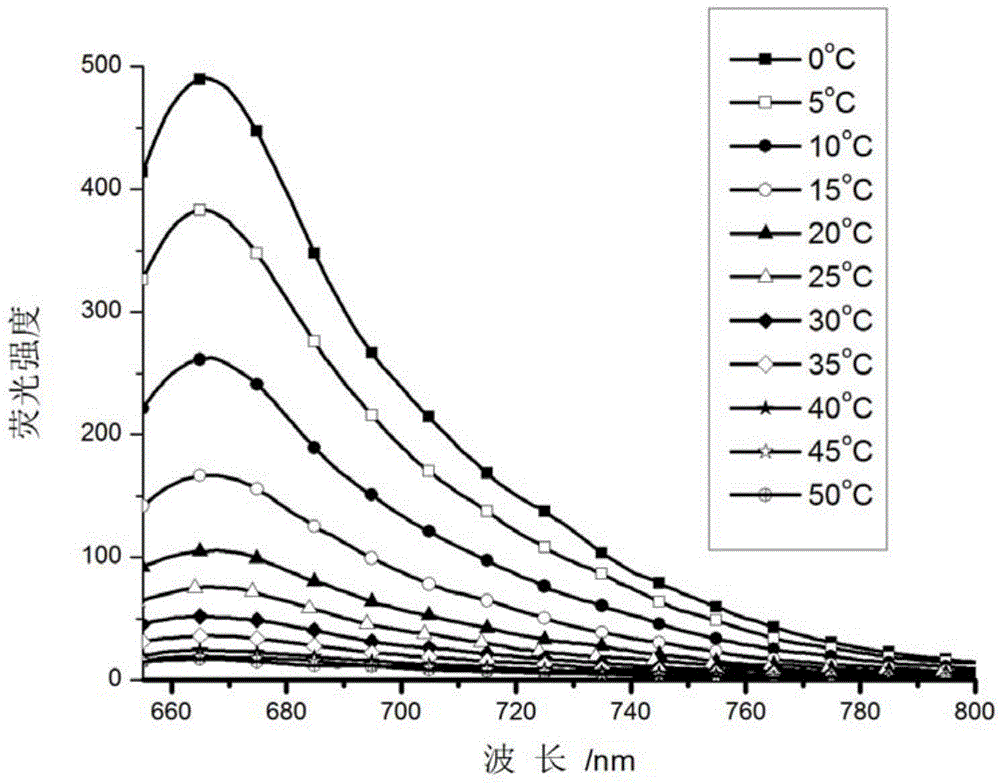
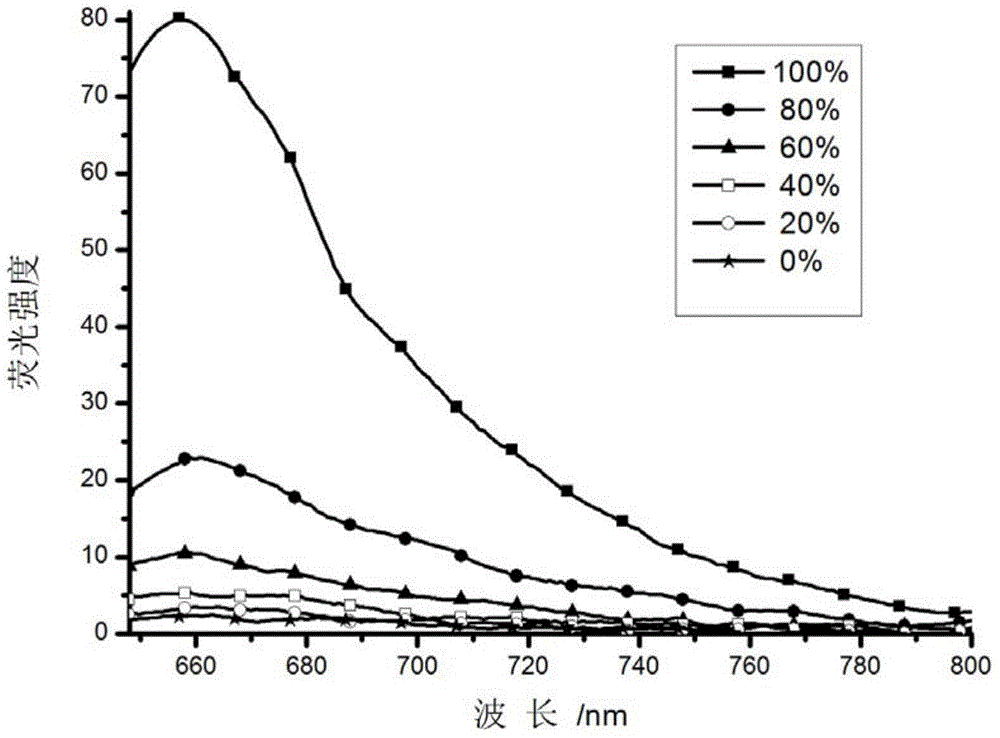
[0043] Prepare 1uM probe solutions with different viscosities (methanol and ethylene glycol 1:0-0:1, V / V, ethylene glycol and glycerol system 1:0-0:1, V / V) for fluorescence spectrophotometry gauge test.
[0044] The results show that when the concentration of methanol is large, that is, the viscosity is small, the probe has no fluorescence, and with the continuous increase of the solution viscosity, the fluorescence of the probe increases extremely, and the fluorescence intensity increases by 2500 times ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com