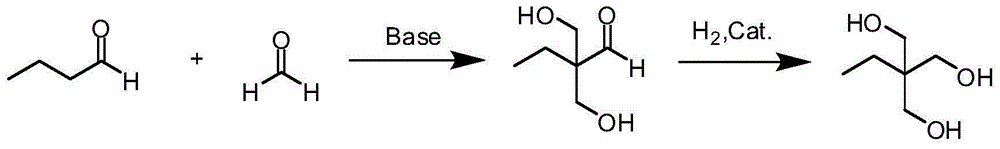
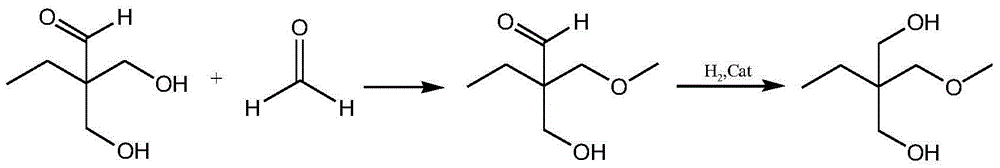
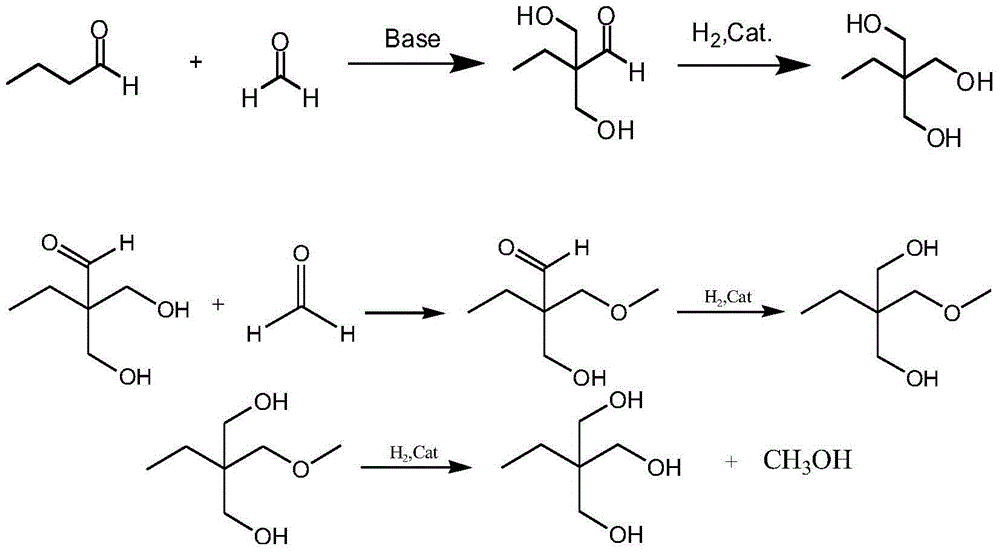
Method for preparing trimethylolpropane by adopting hydrogenation method
A technology of trimethylolpropane and hydrogenation method, which is applied in the direction of condensation preparation of carbonyl compounds, chemical instruments and methods, and preparation of hydroxyl compounds. Catalyst cost is high, achieving considerable economic benefits, high on-line reduction convenience, and improved active surface area and strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 267.3g aluminum nitrate (Al(NO 3 ) 3 ), 150.4 grams of copper nitrate (Cu(NO 3 ) 2 ), 47.4g magnesium nitrate (Mg(NO 3 ) 2 ), 31.9g lanthanum nitrate (La(NO 3 ) 3 ), 3.8g molybdenum nitrate (Mo(NO 3 ) 3 ), 4.4g zirconium nitrate (Zr(NO 3 ) 4 ) after mixing, add water to make 2000 ml of solution and put it into a reaction kettle with stirring and heating function, slowly add 2L of 30wt% sodium carbonate aqueous solution into the reaction kettle, control the reaction temperature at 80° C. for 1 hour, and the reaction process requires pH=9 , aged at 60°C for 2h to obtain a suspension, centrifuged the suspension, washed the solid phase with deionized water until the sodium content was lower than 0.25wt%, dried at 90°C for 48h, added 12.8g of sodium pyrophosphate, and placed Calcined at 600°C for 2 hours, and pressed into tablets to obtain hydrogenation catalyst 1 with a total catalyst weight of 163.1 grams.
Embodiment 2
[0039] 225.5g aluminum nitrate (Al(NO 3 ) 3 ), 228.6g copper nitrate (Cu(NO 3 ) 2 ), 10.5g calcium nitrate (Ca(NO 3 ) 2 ), 42.9g cerium nitrate (Ce(NO 3 ) 3 ), 2.1g molybdenum nitrate (Mo(NO 3 ) 3 ), 7.4g zirconium nitrate (Zr(NO 3 ) 4 ) after mixing, add water to form 2000 ml of solution and put it into a reactor with stirring and heating function, slowly add 2L of 30wt% potassium carbonate aqueous solution into the reactor, control the reaction temperature at 90° C. for 1 hour, and the reaction process requires pH=10 , aged at 60°C for 2h to obtain a suspension, centrifuged the suspension, washed the solid phase with deionized water until the sodium content was lower than 0.25wt%, dried at 100°C for 36h, added 23.4g of sodium pyrophosphate, and placed Calcined at 650° C. for 1 hour, and pressed into pellets to obtain hydrogenation catalyst 2 with a total catalyst weight of 195.9 grams.
Embodiment 3
[0041] 334.1g aluminum nitrate (Al(NO 3 ) 3 ), 135.4g copper nitrate (Cu(NO 3 ) 2 ), 15.2g rubidium nitrate (RbNO 3 ), 15.8g praseodymium nitrate (Pr(NO 3 ) 3 ), 7.5g molybdenum nitrate (Mo(NO 3 ) 3 ), 4.4g zirconium nitrate (Zr(NO 3 ) 4 ) after mixing, add water to make 2000 ml of solution and put it into a reaction kettle with stirring and heating function, slowly add 2L of 30wt% potassium carbonate aqueous solution into the reaction kettle, control the reaction temperature at 85° C. for 1 hour, and the reaction process requires pH=9 , aged at 60°C for 2h to obtain a suspension, centrifuged the suspension, washed the solid phase with deionized water until the sodium content was lower than 0.25wt%, dried at 90°C for 48h, added 6.4g of sodium polyphosphate, and placed Calcined at 550°C for 4 hours, and pressed into pellets to obtain hydrogenation catalyst 3 with a total catalyst weight of 167.0 grams.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com