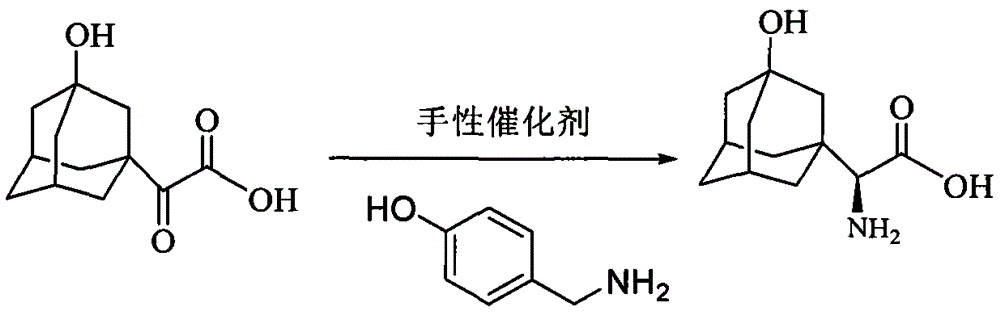
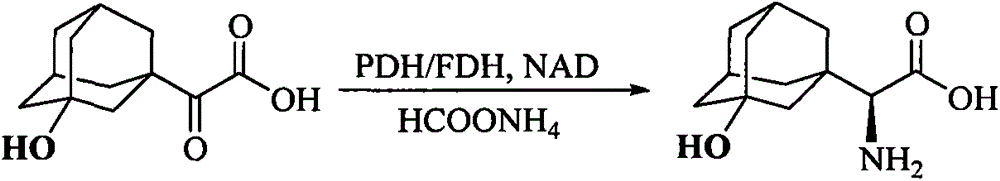The preparation method of (s)-3-hydroxyadamantane glycine
A technology of adamantane glycine and adamantane, which is applied in the field of preparation of -3-hydroxyadamantane glycine, can solve the problems of low enantioselectivity, high cost of biocatalytic enzymes, unsuitable for domestic production and the like, and achieves enantiomeric The effect of high bioselectivity, pollution-free industrial production, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
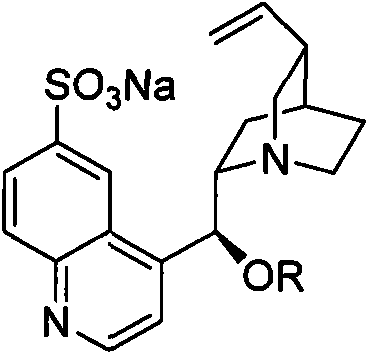
[0021] Preparation of quinoline type alkaloid cinchonine sulfonate derivatives:
[0022] In a 250ml two-necked flask, add cinchonine (3.1g) and solvent chloroform (50ml) where R is methyl, stir to dissolve and cool to below 10°C with ice water, then slowly add chlorosulfonic acid (1.2g) in chloroform Solution, reaction control below room temperature, stirring reaction, TLC monitoring reaction is complete, add sodium bicarbonate (3g) aqueous solution, stir for 3 hours, evaporate part of solvent under reduced pressure, then add sodium bicarbonate solution and carry out liquid separation, collect water phase , part of the water was distilled off under reduced pressure, cooled and crystallized to obtain 1.3 g of the product. MS(EI): 411.14.
[0023] 1 H NMR: 1.13-1.50(2H), 1.69-1.78(3H), 2.16(1H), 2.60(1H), 2.62(1H), 3.05(1H), 3.11(1H), 3.51(1H), 3.60(3H ), 3.92(1H), 4.90(1H), 4.95(1H), 5.49(1H), 5.72(1H), 7.44(1H), 7.46(1H), 7.56(1H), 7.92(1H), 8.45(1H ).
[0024] Compounds ...
Embodiment 1
[0026] Add 2-(3-hydroxyl-1-adamantane)-2-oxoacetic acid (20.0g, 89mmol) and quinoline alkaloid cinchonine sulfonate (0.6g) successively in a 250ml two-necked flask , 60g of 5% aqueous sodium hydroxide solution and p-hydroxybenzylamine (15.4g, 0.125mol) mixture was heated up to 40°C, concentrated after 12 hours of reaction, reacted with concentrated hydrochloric acid to adjust the pH value of the reaction solution to about 6, and then frozen to -5°C The product was precipitated left and right, and 19.69 g of the product was obtained by freeze-drying after suction filtration, the ee value was 97.1%, and the yield was 98.2%.
Embodiment 2
[0028] Add 2-(3-hydroxyl-1-adamantane)-2-oxoacetic acid (20.0g, 89mmol) and quinoline alkaloid cinchonine sulfonate (0.8g ), 5% aqueous sodium hydroxide solution 80g and p-hydroxybenzylamine (15.4g, 0.125mol), the mixture was heated up to 40°C, concentrated after 12 hours of reaction, reacted with concentrated hydrochloric acid to adjust the pH value of the reaction solution to about 6 and then frozen to - The product was precipitated at about 5°C, and 19.4 g of the product was obtained by freeze-drying after suction filtration, with an ee value of 98.5% and a yield of 96.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com