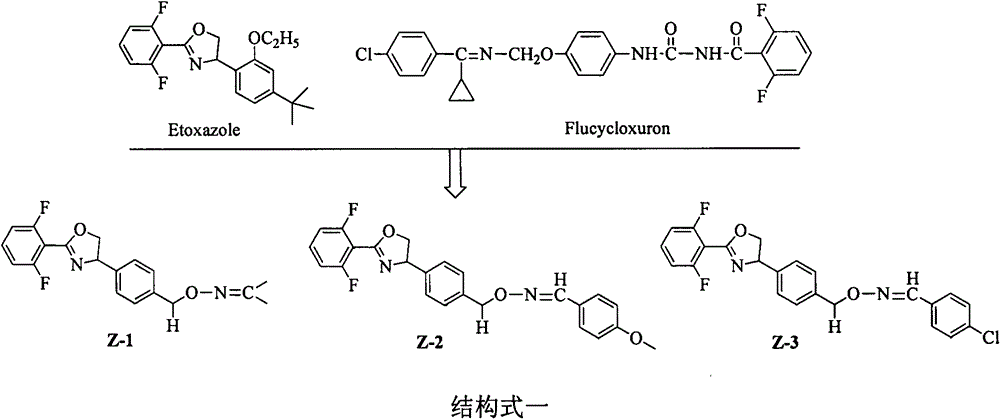
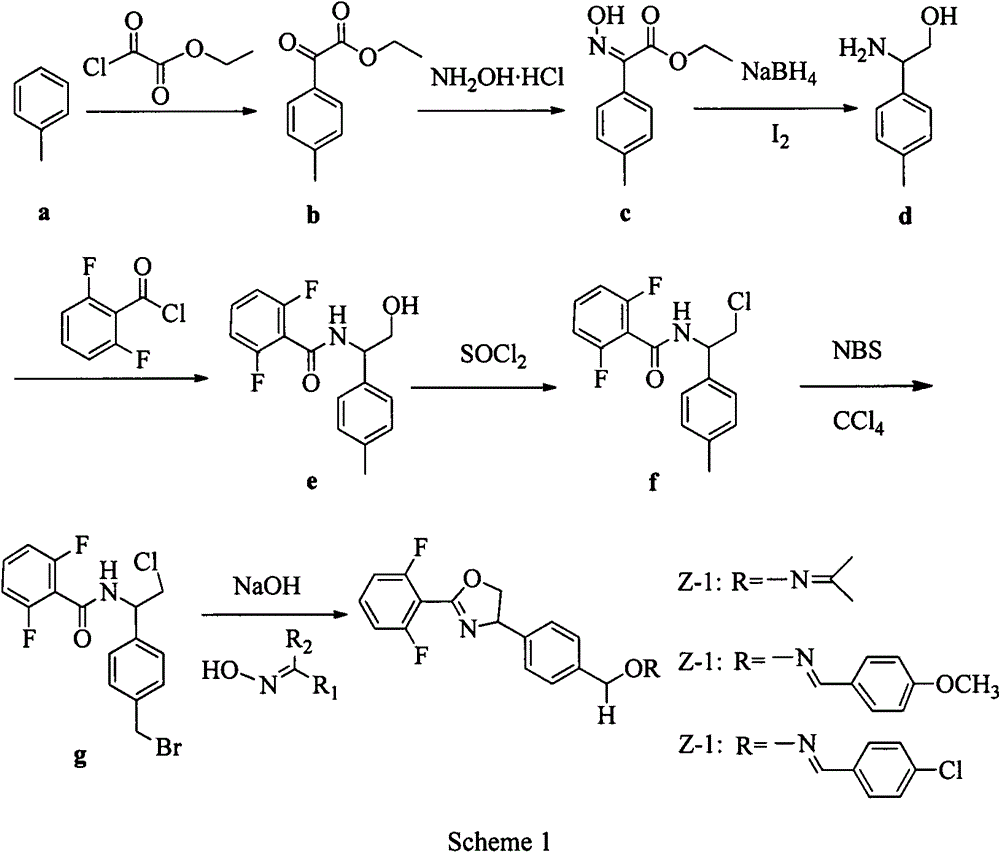
4-phenyl para-alcohol oxime ether-containing oxazoline compound and preparation and application in controlling insects, mites, bacteria and weeds
A technology of alcohol oxime ether and oxazoline, which is applied in the field of oxazoline compounds, can solve the problems of destroying ecological balance, environmental pollution resistance, and polluting the environment, and achieve low toxicity, good environmental compatibility, and wide biological spectrum Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of Compound 1:
[0026] In a 250 mL single-neck flask, 5.0 g (16.1 mmol) of compound f, 40 mL of dichloromethane, 40 mL of water, 1.9 g (16.1 mmol) of potassium bromide, and 1.33 g (8.05 mmol) of potassium bromate were sequentially added. Control the temperature in an ice-water bath to 0℃, add dropwise 20 mL of a solution containing 6.1 mL of concentrated hydrochloric acid, and react for 25 hours. TLC monitors the completion of the reaction and separates the layers. The organic phase is washed with sodium carbonate solution and sodium bicarbonate solution until it is close to colorless, and then used Extract the aqueous phase with dichloromethane, combine the organic phases and wash with sodium chloride solution, anhydrous MgSO 4 It was dried, filtered with suction, and spin-dried to obtain a white solid, which was recrystallized with toluene and petroleum ether to obtain the product g, 3.5 g in total, with a yield of 55.9%. Melting point: 127-128°C. 1...
Embodiment 2
[0028] Example 2: Synthesis of Compounds 2-11:
[0029] Compounds 2-11 were synthesized using the method shown in Example 1.
[0030] Compound 2
[0031] Yellow thick liquid with a yield of 40%. 1 H NMR(400M, CDCl 3 )7.46-7.41(m, 1H), 7.36(d, J=8.0Hz, 2H), 7.32(d, J=8.0Hz, 2H), 7.00(t, J=8.4Hz, 2H), 5.52-5.42( m, 1H), 5.05 (s, 2H), 4.85-4.78 (m, 1H), 4.32-4.28 (m, 1H), 2.50 (t, J=6.2, 2H), 2.24-2.15 (m, 2H), 1.64-1.66 (m, 4H), 1.59-1.61 (m, 2H). HRMS (ESI): Calcd.for C 22 H 22 F 2 N 2 O 2 H[M+H] + 385.1722; found385.1722.
[0032] Compound 3
[0033] Light yellow liquid, the yield was 46.4%. 1 H NMR(400MHz, CDCl 3 )δ7.46-7.41(m, 1H), 7.37(d, J=8.0Hz, 2H), 7.32(d, J=8.0Hz, 2H), 7.00(t, J=8.0Hz, 2H), 5.47( dd, J = 10.4, 8.0 Hz, 1H), 5.06 (s, 2H), 4.81 (dd, J = 10.4, 8.4 Hz, 1H), 4.30 (t, J = 8.4 Hz, 1H), 2.59-2.54 (m , 2H), 2.38-2.34 (m, 2H), 1.73-1.56 (m, 8H). HRMS (MALDI): Calcd.for C 21 H 20 F 2 N 2 O 2 [M+H] + 399.1879; found399.1875.
[0034] Compound 4
[0035] Yellow thick liquid...
Embodiment 3
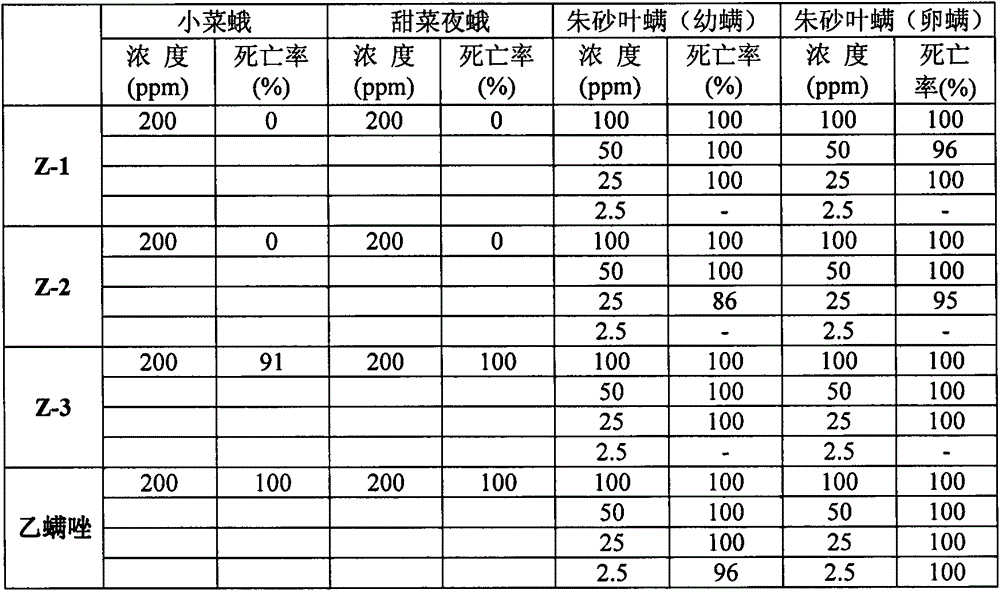
[0050] Example 3: 4-phenyl para-position of oxazoline compounds containing alcohol oxime ether structure 1-11 to armyworm (M. separate), Culex pipiens (C.pipiens), and cotton bollworm (H. armigera) And the insecticidal activity of corn borer (P.nubilalis):
[0051] The measurement procedure is as follows:
[0052] Activity Test of Oriental Armyworm
[0053] The experimental method of the oriental armyworm: the leaf dipping method, after configuring to the required concentration, immerse the leaf with a diameter of about 5-6cm in the liquid medicine for 5-6 seconds, take it out, dry on absorbent paper, and place it in the designated In the petri dish, insert 10 3rd instar larvae, put them in the insect breeding room at 27±1℃ and observe the results after 3-4 days.
[0054] Viability test of mosquito larvae
[0055] The experimental method of mosquito larvae: Culex pipiens pallens, a normal population raised indoors. Weigh about 5 mg of the test compound into a penicillin vial, add 5 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com