Preparation method for graphene-coated metal nanometer particle catalyst and application of graphene-coated metal nanometer particle catalyst
A technology coated with metal nanoparticles and graphene, applied in chemical instruments and methods, physical/chemical process catalysts, nanotechnology, etc., can solve the problems of poor stability of Pt-based electrocatalysts, easy agglomeration of metal nanoparticles, and low degree of graphitization and other problems, to achieve the effects of large-scale production, simple and effective operation method, and high electrocatalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
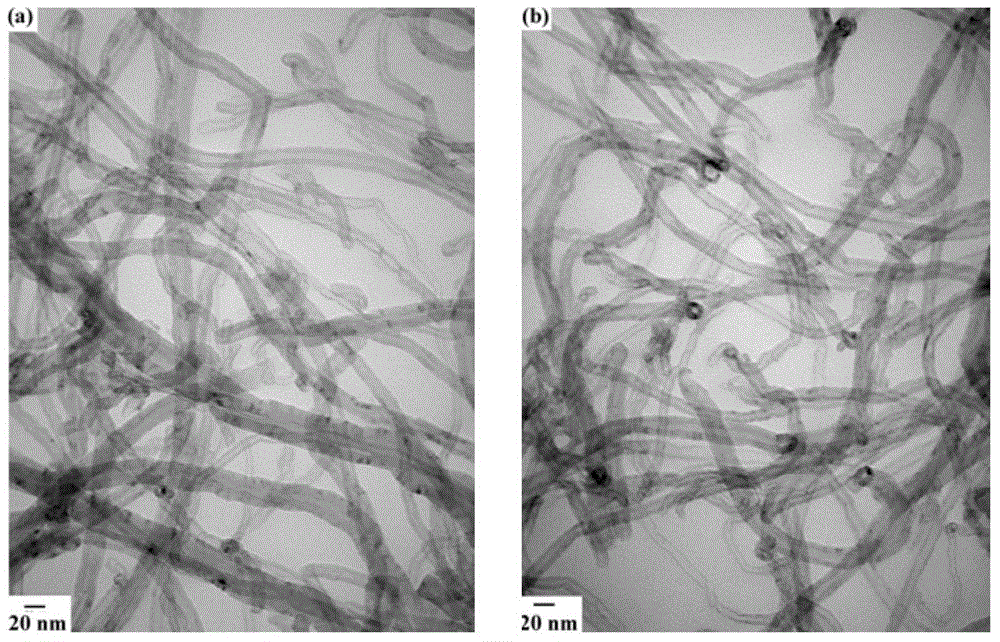
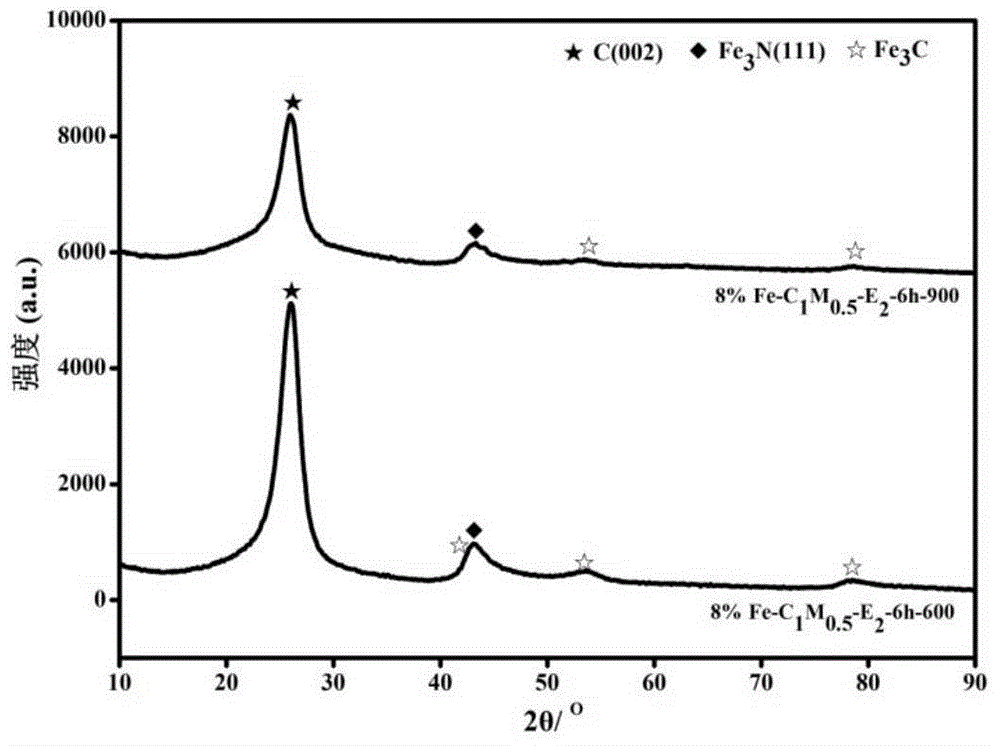
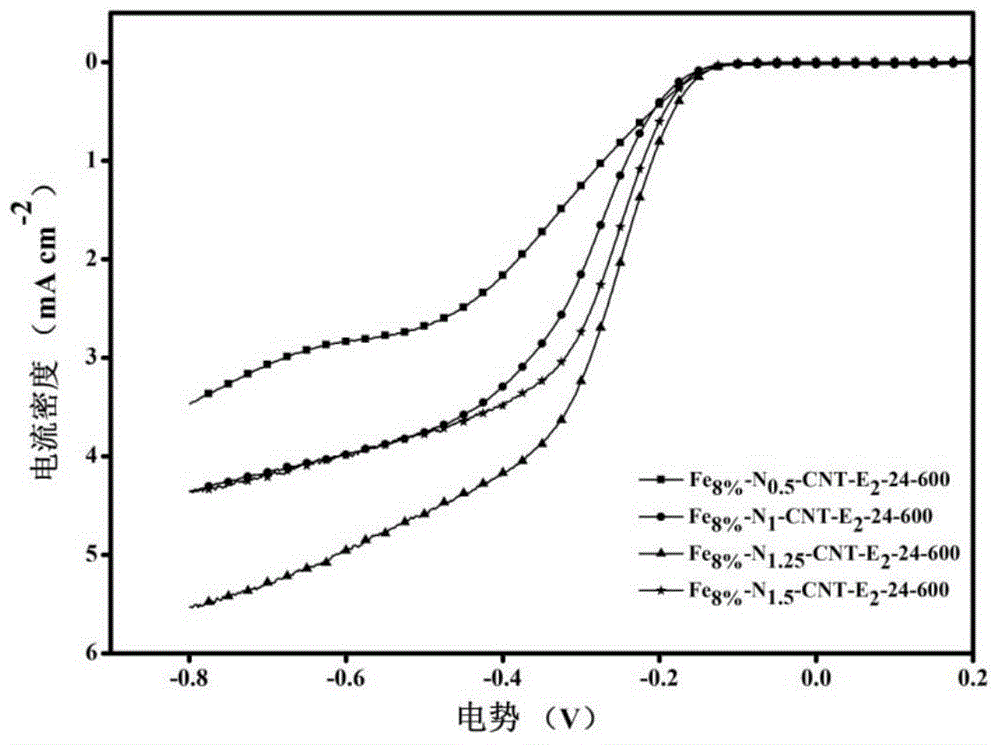
Image
Examples
Embodiment 1
[0036] Example 1: Fe 8% -N 0.5 -CNT-E 2 -24-600(Fe 8% Refers to the mass content of Fe in the material is 8%, N 0.5 Refers to the quality of nitrogen source melamine is 0.5 times that of carboxylated carbon nanotubes, E 2 Means that the mass of ethylenediaminetetraacetic acid disodium salt is twice that of iron salt, 24 means that the hydrothermal reaction time is 24h, and 600 means that the calcination temperature is 600℃)
[0037] Take 0.1000g of carboxylated carbon nanotubes, 0.0386g of ferric chloride and 0.0500g of melamine and add them to a mixed solution of 30mL of water and ethanol, sonicate for 30min to make the dispersion uniform, then magnetically stir at 50℃ for 2h, and then take 0.0772g of ethylenediamine Tetraacetic acid disodium salt and 10 mL of methanol were added to the above mixed solution and hydrothermally reacted at 150℃ for 24h, washed, filtered, and dried under vacuum at 80℃ to obtain composite Fe 8% -N 0.5 -CNTEDTA-24;
[0038] Put the above materials in N 2...
Embodiment 2
[0039] Example 2: Fe 8% -N 1 -CNT-E 2 -24-600(Fe 8% Refers to the mass content of Fe in the material is 8%, N 1 Means that the quality of nitrogen source melamine is twice that of carboxylated carbon nanotubes, E 2 Means that the mass of ethylenediaminetetraacetic acid disodium salt is twice that of iron salt, 24 means that the hydrothermal reaction time is 24h, and 600 means that the calcination temperature is 600℃)
[0040] Add 0.1000g carboxylated carbon nanotubes, 0.0386g ferric chloride and 0.1000g melamine to a mixed solution of 30mL water and ethanol, sonicate for 30min to make the dispersion uniform, then magnetically stir at 50℃ for 2h, then take 0.0772g ethylenediamine Tetraacetic acid disodium salt and 10 mL of methanol were added to the above mixed solution and hydrothermally reacted at 150℃ for 24h, washed, filtered, and dried under vacuum at 80℃ to obtain composite Fe 8% -N 1 -CNTEDTA-24;
[0041] Put the above materials in N 2 3℃ min under the atmosphere -1 The rate o...
Embodiment 3
[0042] Example 3: Fe 8% -N 1.25 -CNT-E 2 -24-600(Fe 8% Refers to the mass content of Fe in the material is 8%, N 1.25 Refers to the quality of nitrogen source melamine is 1.25 times that of carboxylated carbon nanotubes, E 2 Means that the mass of ethylenediaminetetraacetic acid disodium salt is twice that of iron salt, 24 means hydrothermal time reaction is 24h, 600 means calcination temperature is 600℃)
[0043] Take 0.1000g of carboxylated carbon nanotubes, 0.0386g of ferric chloride and 0.1250g of melamine into a mixed solution of 30mL of water and ethanol, sonicate for 30min to make the dispersion uniform, then magnetically stir at 50℃ for 2h, and then take 0.0772g of ethylenediamine Tetraacetic acid disodium salt and 10 mL of methanol were added to the above mixed solution and hydrothermally reacted at 150℃ for 24h, washed, filtered, and dried under vacuum at 80℃ to obtain composite Fe 8% -N 1.25 -CNTEDTA-24;
[0044] Put the above materials in N 2 3℃ min under the atmosphere ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com