Palladium ion fluorescent probe, and preparation method and applications thereof
A fluorescent probe and palladium ion technology, applied in the field of dye chemistry, can solve problems such as low detection wavelength, difficulty in eliminating environmental interference, and easy damage to cells and tissues, and achieve high yield, good structural stability of dyes, and easy The effect of separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Synthesis of compound III
[0027] Dissolve isophorone I (5.0g, 36.2mmol) and malononitrile II (2.87g, 43.4mmol) in 50ml of ethanol, and then add piperidine (0.5g, 4.8mmol), at 120°C under nitrogen protection Heat to reflux for 12 hours. After the reaction, the solvent is evaporated to dryness, dissolved in dichloromethane, washed with water, dried over anhydrous sodium sulfate, filtered with suction, and rotary evaporated under reduced pressure to obtain the crude product of formula III; after separation and purification on a silica gel column, compound III 4.72 g, the productive rate is 70%. The eluent is: petroleum ether:ethyl acetate=5:1
[0028]
[0029] (2) Synthesis of Compound V
[0030] Dissolve (3,5,5-trimethylcyclohex-2-enylidene)malononitrile (compound III) (300mg, 1.6mmol) and p-acetamidobenzaldehyde (compound IV) (263mg, 1.6mmol) In 20 milliliters of toluene, 0.3 milliliters of piperidine and acetic acid were respectively added as catalysts, and ...
Embodiment 2
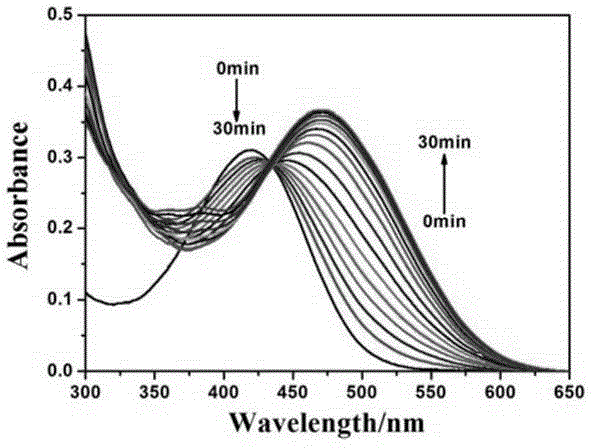
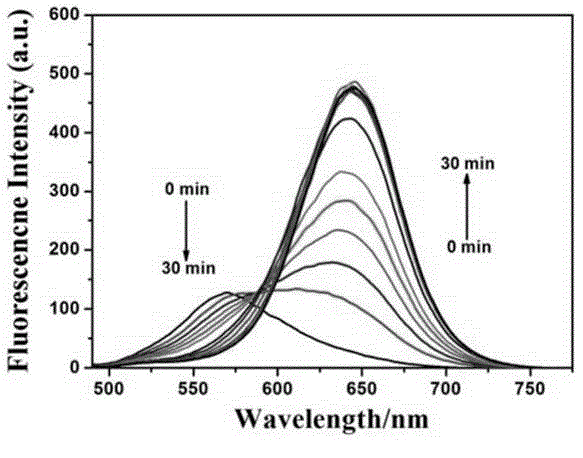
[0038] Embodiment 2, probe compound VII to Pd (PPh 3 ) 4 response time experiment
[0039] Using the compound VII synthesized in Example 1, use DMSO to make 1×10 -4 mol / L of the probe mother solution, and then add 0.3 ml of the mother solution to a mixed solution of 1.5 ml of PBS buffer (20 mM) and 1.2 ml of DMSO. Add 50 μM Pd(PPh 3 ) 4 Detect the change of its fluorescence intensity and ultraviolet absorbance within 0-30min. The slit during the fluorescence spectrum test was set to 5 / 5. Test results such as figure 1 and figure 2 It can be seen that with the increase of adding time, the ultraviolet absorbance at 420nm decreases continuously, while the fluorescence intensity at 472nm increases continuously, while the fluorescence intensity at 570nm decreases continuously, and the fluorescence intensity at 643 increases continuously. Both groups of experiments could achieve response saturation within 30 minutes.
Embodiment 3
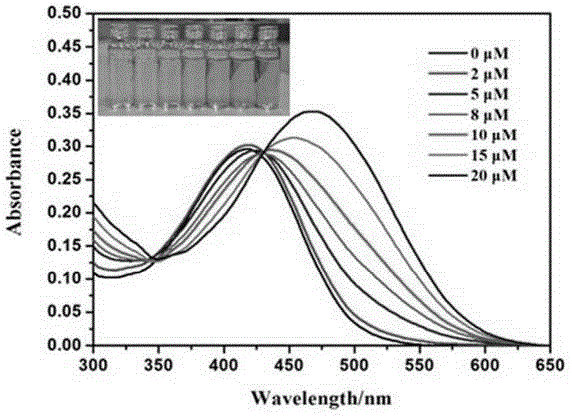
[0040] Embodiment 3, probe compound VII to Pd (PPh 3 ) 4 concentration titration experiment.
[0041] Compound VII synthesized in Example 1 was prepared as a solution according to Example 2 and diluted into a mixed solution of 1.5 ml of PBS buffer (20 mM) and 1.2 ml of DMSO. Add 0, 2, 5, 7, 10, 15, 20 μM Pd(PPh 3 ) 4 , Wait for 30 minutes for each addition and then detect the changes of its UV absorbance and fluorescence intensity. The slit during the fluorescence spectrum test was set to 5 / 5. From image 3 and Figure 4 It can be seen that with Pd(PPh 3 ) 4 As the concentration increases, the UV absorbance at 420nm decreases continuously, while the UV absorbance at 472nm increases continuously. At the same time, the fluorescence intensity at 570nm decreases continuously, and the fluorescence intensity at 643 increases continuously. The ultraviolet red shift of two groups of experiments reached 52nm, and the fluorescence red shift reached 73nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com