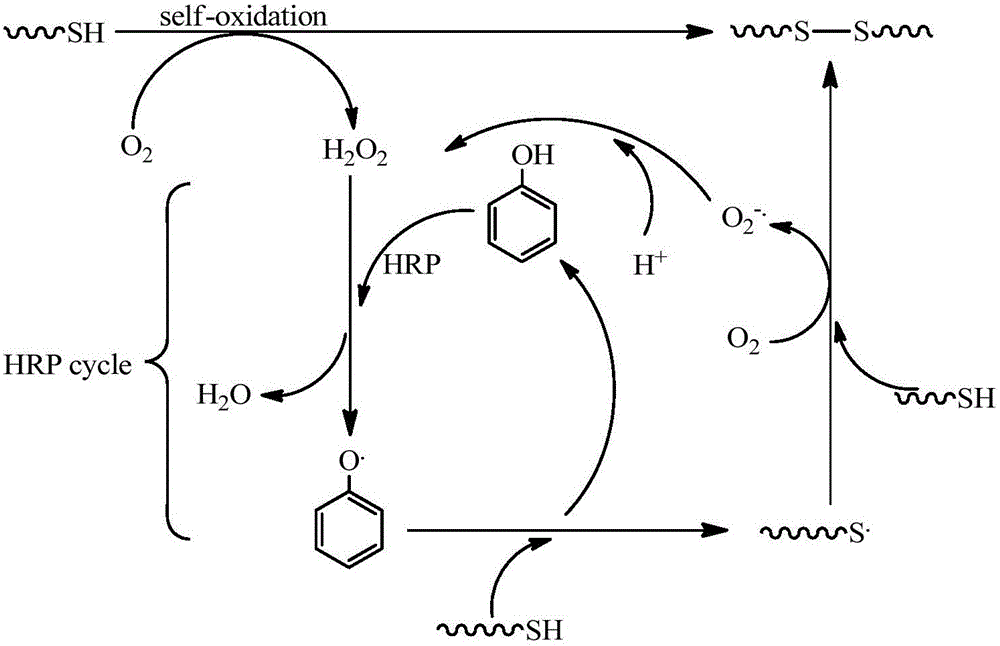
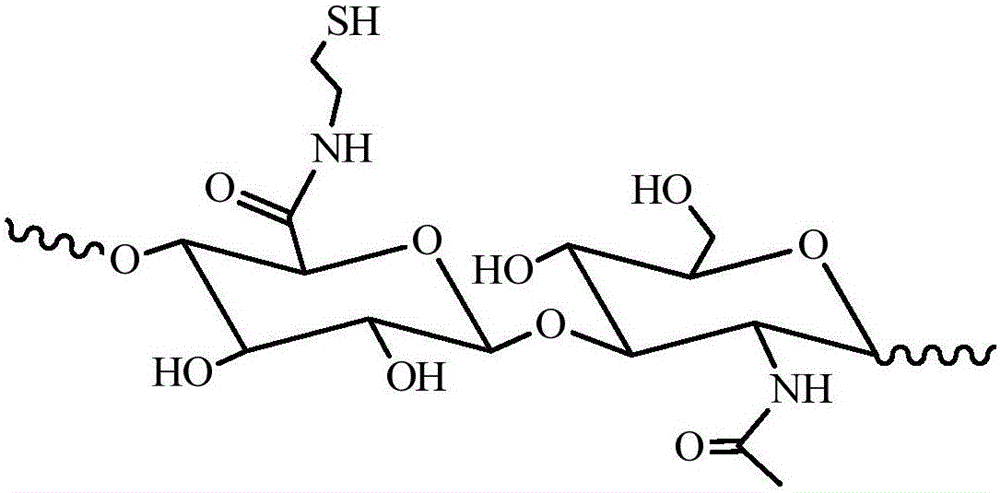
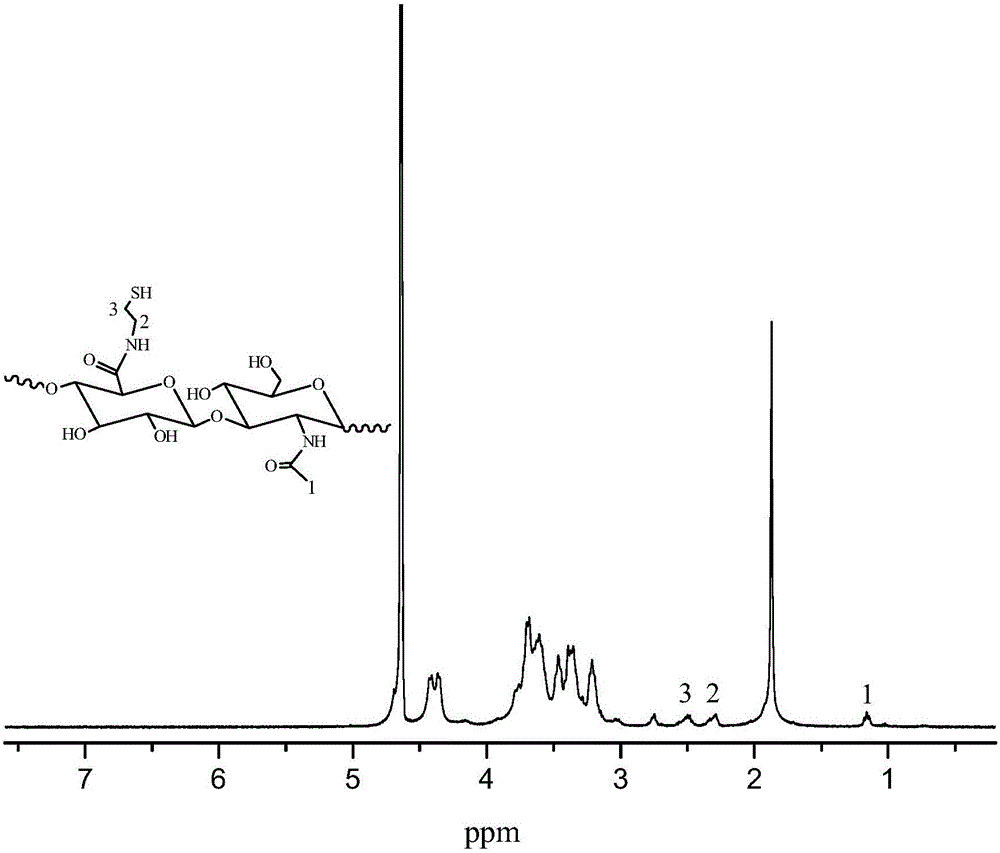
Enzyme-catalyzed disulfide bond-crosslinked natural polymer hydrogel and preparation method thereof
An enzyme-catalyzed disulfide bond, natural polymer technology, applied in the field of medical biomaterials, can solve the problems of poor hydrogel homogeneity, reduced enzyme activity, affecting the activity of loaded drugs, etc., and achieves fast cross-linking speed, simple operation, excellent Effects of Biocompatibility and Biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of hyaluronic acid macromolecule (HA-SH) grafted with sulfhydryl group.
[0040] Weigh sodium hyaluronate (2g, 5mmol) and dissolve it in 100 mL of double distilled water, stir with a magnetic stirrer at room temperature until it is completely dissolved. Weigh N-hydroxysuccinimide (NHS) (2.3g, 20mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC.HCL) (3.832g) , 20mmol) add the above-mentioned completely dissolved sodium hyaluronate solution, adjust the pH value of the solution to about 5.4, and activate at room temperature for 1 hour. Weigh cystamine dihydrochloride (3.372g, 15mmol) and add it to the above activated solution, and stir at room temperature for 48h. The reaction solution was transferred to a dialysis bag (MWCO3500) and dialyzed with double distilled water for 24 hours. Weigh D,L-dithiothreitol (DTT) (4.6275g, 30mmol) into the above-mentioned reaction solution that has been dialyzed for 24 hours, adjust the pH to 8.5, avoid li...
Embodiment 2
[0042] Preparation of sulfhydryl-grafted chitosan macromolecule (CS-SH)
[0043] Weigh chitosan (0.5g) and dissolve it in 50mL of double distilled water, add 2mL of 1mol / L hydrochloric acid, stir with a magnetic stirrer at room temperature until it is completely dissolved, and adjust to pH6 with 1mol / L NaOH. Weigh N-acetylcysteine-L-acid (NAC) (2.3563g, 14.4mmol), N-hydroxysuccinimide (NHS) (2.0g, 17.3mmol), 1-(3-dimethylaminopropyl) Yl)-3-ethylcarbodiimide hydrochloride (EDC.HCL) (3.5g, 18.3mmol) was dissolved in 6mL N,N-dimethylformamide (DMF). The above DMF mixed solution was added dropwise to the completely dissolved chitosan solution, the pH value of the solution was maintained at about 6.0, and the reaction was stirred at room temperature for 24 hours. The reaction solution was transferred to a dialysis bag (MWCO3500) and dialyzed with double distilled water for 24 hours. Weigh D,L-Dithiothreitol (DTT) (2.31g, 15mmol) and add it to the above-mentioned reaction solution th...
Embodiment 3
[0045] Preparation of polyglutamic acid macromolecule (PGA-SH) grafted with sulfhydryl
[0046] Weigh sodium polyglutamate (0.3 g) and dissolve in 30 mL of double-distilled water, and stir with a magnetic stirrer at room temperature until it is completely dissolved. Weigh N-hydroxysuccinimide (NHS) (0.2624g, 2.28mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC.HCL) (1.14) g, 5.94mmol) Add the above-mentioned completely dissolved sodium polyglutamate solution, adjust the pH value of the solution to about 6.0, and activate at room temperature for 1 hour. Weigh cysteamine hydrochloride (0.675 g, 5.94 mmol) and add it to the activated solution, and stir for 24 hours at room temperature. The reaction solution was transferred into a dialysis bag and dialyzed with 1 mM hydrochloric acid for 1 day, 1 wt% NaCl solution for 1 day, and 1 mM hydrochloric acid for 1 day. After dialysis, the reaction solution was quickly frozen with liquid nitrogen and freeze-dried to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com