Preparation method for antimicrobial peptide-lysozyme fusion protein and application thereof
A technology of fusion protein and antibacterial peptide, applied in the fields of peptide/protein components, biochemical equipment and methods, antibacterial drugs, etc., can solve the problems of high construction cost of expression system, different sterilization mechanism, low protein expression, etc. Effects of respiratory disease incidence, survival rate improvement, and diarrhea rate reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
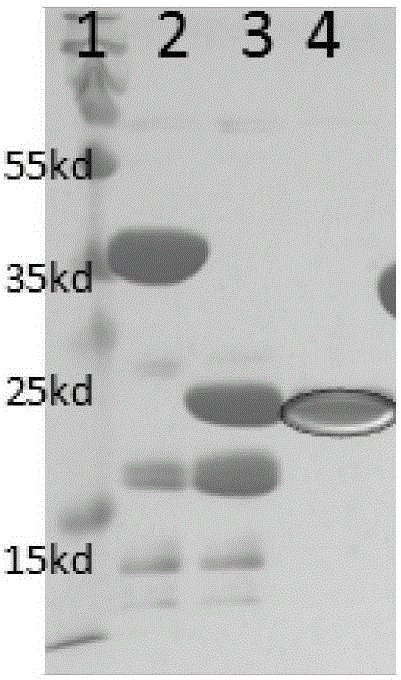
Image
Examples
Embodiment 1
[0025] Example 1: Tandem expression and protein purification of antimicrobial peptide Thanatin and T4 lysozyme
[0026] 1. Design and synthesis of fusion genes
[0027] Human thanatin (thanatian) and T4 lysozyme protein are connected in the form of thanatian-connecting peptide-T4 lysozyme, so the thanatin gene is in front and the T4 lysozyme gene is in the back.
[0028] The connecting peptide is a 3GSA fragment, which is inserted between the antimicrobial peptide and the lysozyme gene, as a flexible peptide, which reduces the structural impact of the two parts of the protein and increases the solubility of the fusion protein expression.
[0029] Adding sumo protein at the N-terminus of the fusion protein not only plays a melting role, but also helps to reduce the activity of the antibacterial protein on the toxicity of the host bacteria, so that it can be expressed in large quantities in the host bacteria.
[0030] The antimicrobial peptide thanatin gene sequence is shown in...
Embodiment 2
[0040] Embodiment 2: Antibacterial activity assay of antimicrobial peptide Thanatin-T4 lysozyme (tha-3GSA-T4) fusion protein
[0041] The antibacterial activity of the fusion protein was detected by the Oxford cup method:
[0042] Pour a layer of agar powder into the plate first, and after it solidifies, add 0.1% of the test bacteria in the logarithmic growth phase to 20m of LB solid medium at about 50°C, mix well, and spread the plate. Put an Oxford cup vertically on the plate, add 200 μL of sample into it, and put it in an oven at 37° C. for 12 hours. Thanatin and T4 lysozyme were used as controls alone.
[0043] The test bacteria include Gram-positive bacteria and Gram-negative bacteria, wherein Bacillus subtilis (ATCC6633) is selected as Gram-positive bacteria, and Escherichia coli (ATCC25922) is selected as Gram-negative bacteria.
[0044] The strength of the antibacterial activity was judged by the size of the inhibition zone.
[0045] The result is as image 3 , F...
Embodiment 3
[0046] Example 3: Study on the survival rate of piglets by tha-3GSA-T4 fusion protein
[0047] The breeds used in the experiment were triple hybrids of Duroc, Yorkshire and Landrace sows. During the experiment, all piglets were vaccinated according to the normal immunization program and managed in a conventional way.
[0048] Test group: tha-T4 fusion protein was added to the feed at a ratio of 3‰ (weight ratio, the same below). Piglets are added from the 7-day-old crevice until they are released from the nursery.
[0049] Control group: Piglets were added Thanatin, T4 lysozyme, Thanatin+lysozyme (thanatin and T4 lysozyme mixed in equimolar ratio), and conventional antibiotic amoxicillin in the feed at a ratio of 3‰.
[0050] The results are shown in Table 1.
[0051] Table 1 Statistics on the survival rate of piglets in lactation and nursery stages
[0052]
[0053] It can be seen from Table 1 that the tha-T4 fusion protein can significantly improve the survival rate of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com