Method for preparing modified lithium nickel manganese oxide which is anode material of lithium ion battery
A technology of lithium ion battery and cathode material, which is applied in the field of preparation of modified nickel manganate, can solve the problem of poor cycle performance, thermal stability and high temperature performance, poor battery safety and thermal stability, and difficulty in realizing cobalt Lithium oxide replacement and other issues, to achieve the effect of improving cycle performance and high temperature stability, improving high pressure cycle performance and high temperature stability, and good market promotion value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
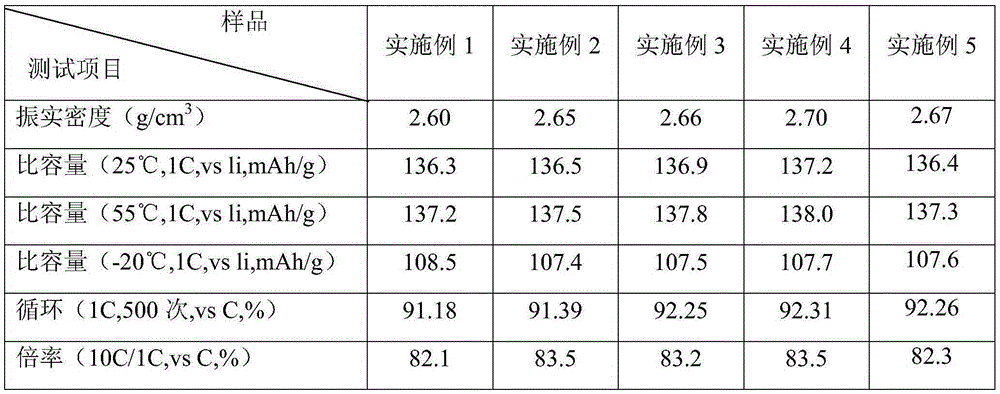
Embodiment 1
[0036] (1) Preparation of manganese salt and nickel salt materials: mix manganese sulfate and nickel sulfate materials with a Mn:Ni molar ratio of 3:1;
[0037] (2) The sol-gel method prepares the nickel-manganese precursor: the mixture obtained in step (1) is added in the chelating agent polyethylene glycol-1000, and the quality of the chelating agent is 120% of the metal Mn+Ni quality, with ammonium bicarbonate Adjust the pH to 8-10, stir until viscous, add pure water under stirring to make a solution with a metal Mn+Ni ion concentration of 100g / l, add a sedimentation aid hydroxyl Methyl cellulose until the precipitation is complete, filter, and dry the precipitate to obtain a nickel-manganese precursor;
[0038](3) Three-dimensional oblique mixing: use a three-dimensional oblique mixer to disperse and mix the nickel-manganese precursor and lithium carbonate salt obtained in step (2) for 2 hours under the medium of polyurethane balls, metal (Mn+Ni): Li moles The ratio is 0....
Embodiment 2
[0046] (1) Preparation of manganese salt and nickel salt materials: mix manganese nitrate and nickel nitrate materials with a Mn:Ni molar ratio of 3:1;
[0047] (2) The sol-gel method prepares the nickel-manganese precursor: the mixture obtained in step (1) is added in the chelating agent polyethylene glycol-4000, and the quality of the chelating agent is 100% of the metal Mn+Ni quality, with ammonium bicarbonate Adjust the pH to 8-10, stir until viscous, add pure water under stirring to make a solution with a metal Mn+Ni ion concentration of 120g / l, add a sedimentation aid hydroxyl that is 5% of the metal Mn+Ni Methyl cellulose until the precipitation is complete, filter, and dry the precipitate to obtain a nickel-manganese precursor;
[0048] (3) Three-dimensional oblique mixing: use a three-dimensional oblique mixer to disperse and mix the nickel-manganese precursor and lithium carbonate salt obtained in step (2) for 4 hours under the medium of zirconia balls, metal (Mn+Ni)...
Embodiment 3
[0056] (1) Preparation of manganese salt and nickel salt materials: mix manganese chloride and nickel chloride materials with a Mn:Ni molar ratio of 3:1;
[0057] (2) Sol-gel method to prepare nickel-manganese precursor: the mixture of step (1) gained is added chelating agent polyethylene glycol-2000, and the quality of chelating agent is 80% of metal Mn+Ni quality, adjusts with ammonium bicarbonate When the pH is 8-10, stir until viscous, add pure water under stirring to make a solution with a metal Mn+Ni ion concentration of 150g / l, and add a settling aid hydroxyformide whose mass is 4% of the mass of the metal Mn+Ni Base cellulose until the precipitation is complete, filter, and dry the precipitate to obtain the nickel-manganese precursor;
[0058] (3) Three-dimensional oblique mixing: use a three-dimensional oblique mixer to disperse and mix the nickel-manganese precursor and lithium carbonate salt obtained in step (2) for 3 hours under the medium of polyurethane balls, me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com