Preparation method of dimethylaminoethyl acrylate and catalyst thereof
A technology of dimethylaminoethyl acrylate and methyl acrylate, applied in the field of preparation method of dimethylaminoethyl acrylate and its catalyst, to achieve high activity, convenient continuous operation and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
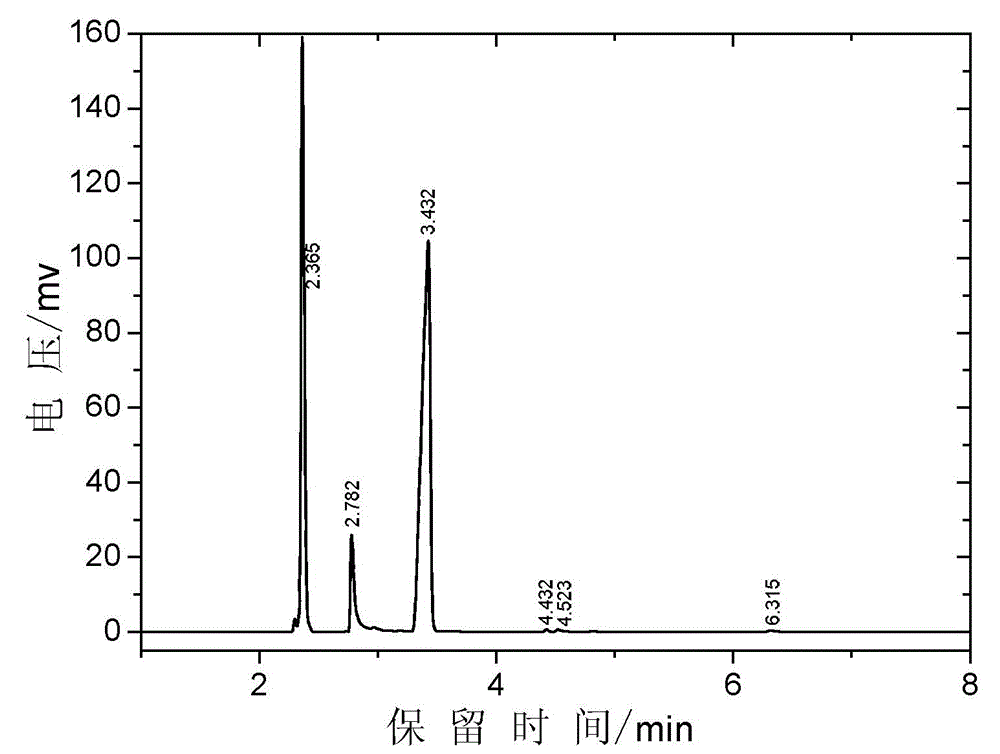
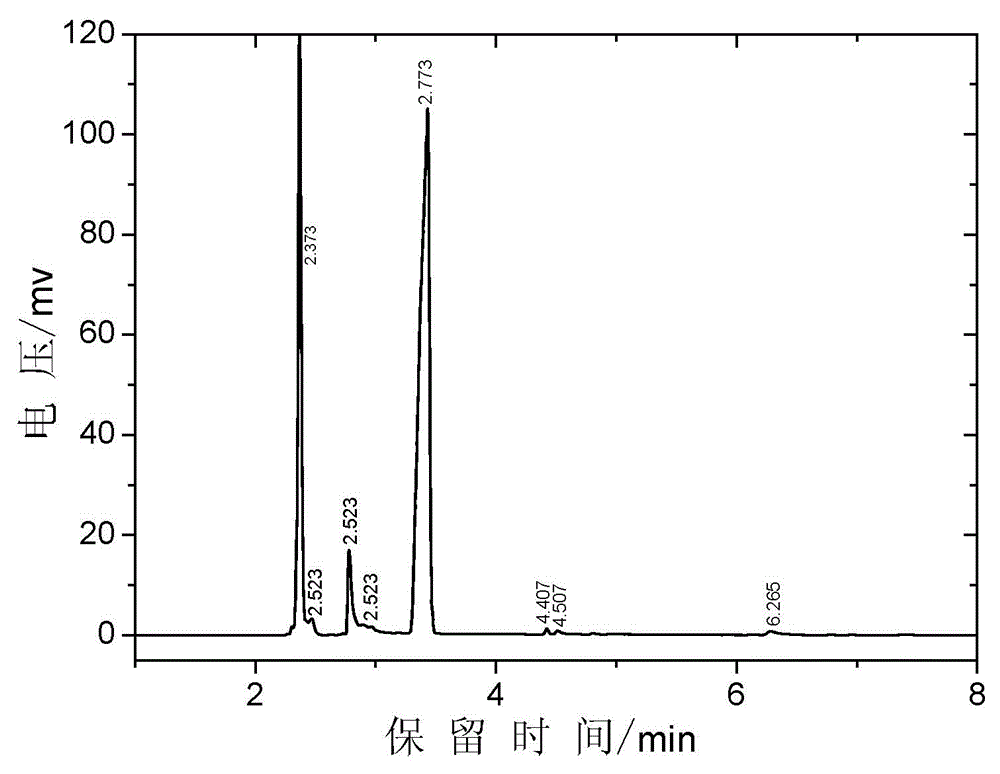
Image
Examples
Embodiment 1
[0040] In a reactor with a packing rectification column and agitator, add a mixture of 260g dimethylaminoethanol, 80g tetraisopropyl titanate and 8.3g ethylene glycol and heat it to reflux, and the formed isopropanol is refined The distillation tower was discharged continuously, and 65g of isopropanol distillate was obtained in 5 hours, and finally 279g of orange liquid product was obtained, which was directly used as a transesterification catalyst, and it consisted of 81.4g of catalyst and 197.6g of dimethylaminoethanol.
Embodiment 2
[0042] Add the catalyst solution of 145g dimethylaminoethanol, 516g methyl acrylate, 48g embodiment 1 (wherein containing catalyzer 14g, the quality of catalyzer is 2% of reaction raw material gross mass) , 1.4g phenothiazine and 0.17g tris-(2,2,6,6-tetramethylpiperidinyl nitroxide free radical) phosphite, stirred, heated to 83~110°C, the temperature at the top of the rectifying column was 62 At ~64°C, methanol is generated during the reaction, which forms an azeotrope with methyl acrylate and is removed. Add dropwise 50g of methyl acrylate solution containing 1% p-hydroxyanisole in the condenser at the top of the rectification column to stabilize the material in the cold rectification column. The conversion rate was monitored by gas chromatographic analysis of the reaction solution in the reaction bottle, and the conversion rate of dimethylaminoethanol reached 94.5% after the transesterification reaction time was 6 hours. After the reaction is completed, lower the temperatur...
Embodiment 3
[0047] Add the catalyst solution of 162g dimethylaminoethanol, 516g methyl acrylate, 24g embodiment 1 (wherein containing catalyst 7g, the quality of catalyst is 1% of reaction raw material gross mass) , 1.4g phenothiazine and 0.17g tris-(2,2,6,6-tetramethylpiperidinyl nitroxide free radical) phosphite, stirred, heated to 83~110°C, the temperature at the top of the rectifying column was 62 At ~64°C, methanol is generated during the reaction, which forms an azeotrope with methyl acrylate and is removed. Add dropwise 50g of methyl acrylate solution containing 1% p-hydroxyanisole in the condenser at the top of the rectification column to stabilize the material in the cold rectification column. The conversion rate was monitored by gas chromatographic analysis of the reaction liquid in the reaction bottle, and the conversion rate of dimethylaminoethanol was 89.0% after the transesterification reaction time was 6 hours. After the reaction is completed, lower the temperature, adjust...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com