Axially chiral enantiomers of drug Lesinurad
An enantiomer and axial chiral technology, applied in the field of R-configuration enantiomer, hyperuricemia and gout, can solve problems such as difficult to develop compounds, no great progress, slow development, etc., to achieve prevention of tissue Abnormal uric acid level symptoms, excellent suppression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Preparation of racemic lesinurad
[0084] Referring to WO2014008295A1, lesinurad was synthesized. Proceed as follows:
[0085]
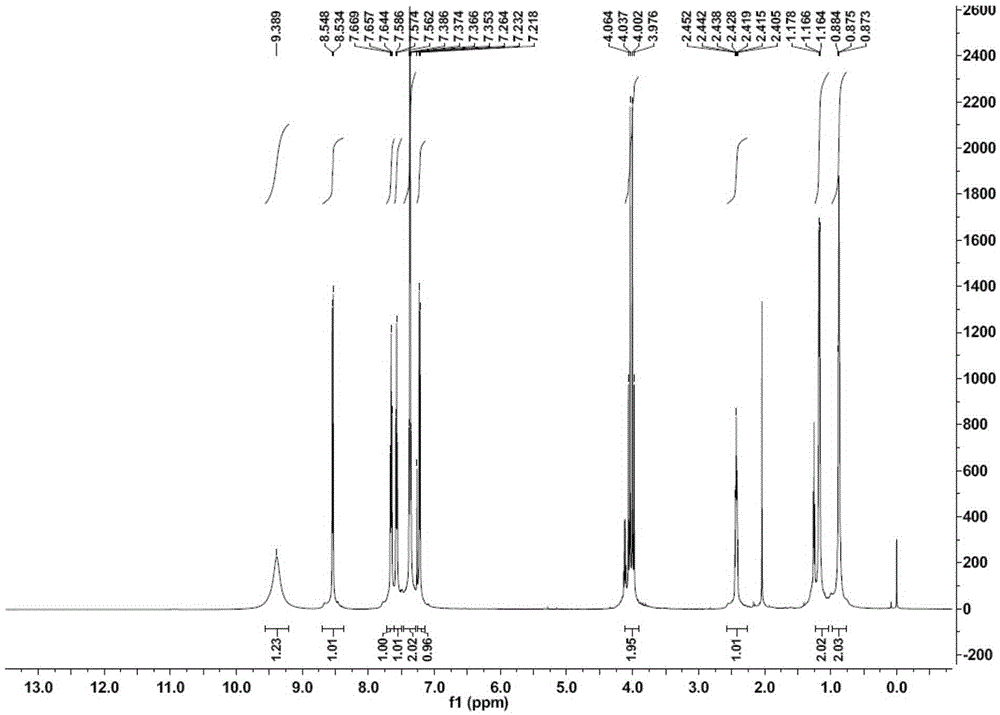
[0086] Add 4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazole-3-thiol (Compound A, 5g, 0.018mol), potassium carbonate (3.74 g, 0.027mol, 1.5eq), DMF (50ml). Ethyl bromoacetate (3.3 g, 0.022 mol, 1.2 eq) was added dropwise with stirring. After dropping, stir the reaction at room temperature for 1.5h. After the reaction of the raw materials was detected by sampling, 100 ml of ice water was dripped into the reaction solution under stirring, and a white solid was precipitated. Stir for 30min, filter with suction and wash with water. After the filter cake was dried, it was recrystallized with ethyl acetate to obtain 4 g of white solid, namely Intermediate B.
[0087] 1HNMR(CDCl3)δ8.54(dd,J=8Hz,1H),8.31(S,1H),7.66(m,1H),7.57(m,1H),7.40(dd,J=8Hz,1H),7.34 (m,2H),4.08(m,2H),3.72(s,3H),2.42(m,1H),1.16(m,2H),0.85(m,2H)
[0088] ESI(M+H): 340 ...
Embodiment 2
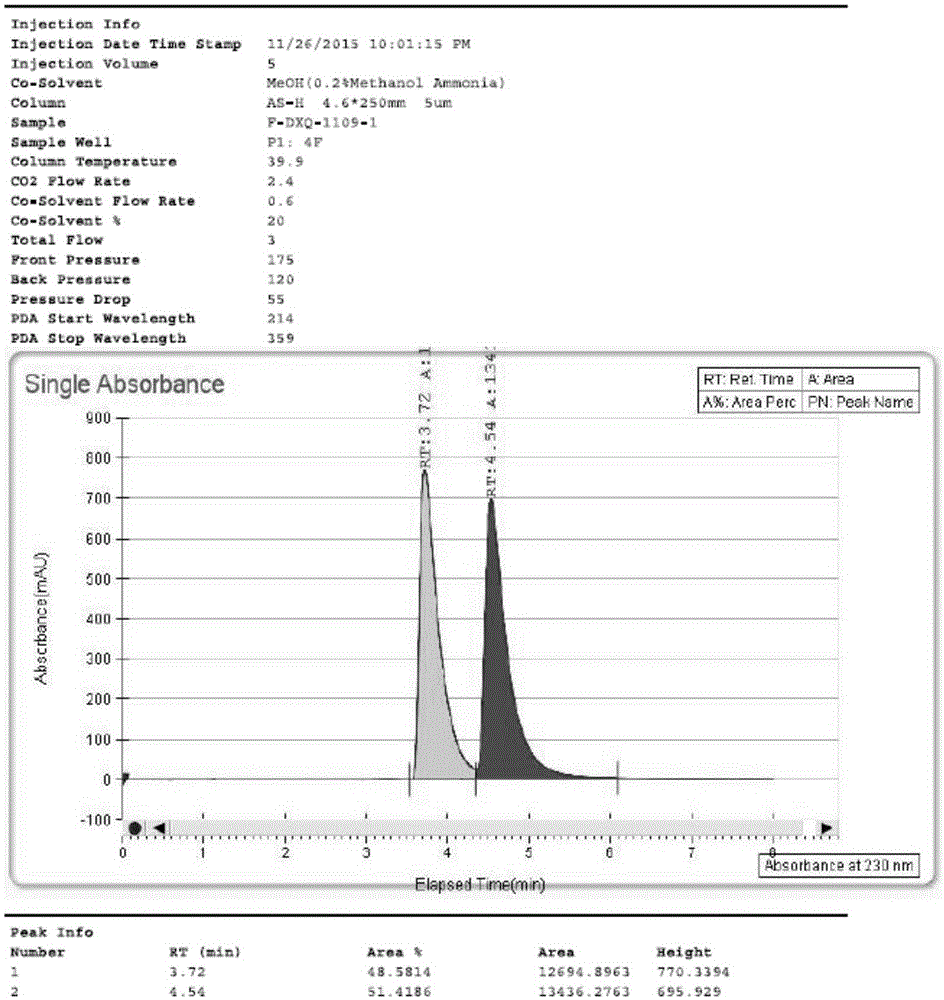
[0097] Preparative liquid phase separation of lesinurad axial chiral enantiomers
[0098] The R configuration axis and the S configuration axis chiral enantiomer of the lesinurad obtained in Example 1 were separated using a preparative liquid chromatograph.
[0099] Instrument: SFC-80 (Thar, Waters), preparative chiral column model: OJ column 20×250mm, 5μm (Dacel),
[0100] Column temperature: 35°C, mobile phase: CO 2 / MeOH=75 / 25, flow rate: 80g / min,
[0101] Detection wavelength: 214nm, column pressure: 100bar, injection volume: 0.2mL,
[0102] Cycle time: 2.5min,
[0103] Sample preparation method: Dissolve 6000mg of racemic lesinurad in 50mL of methanol.
[0104] figure 1 High performance liquid chromatography (HPLC) diagram for the separation of the R-configuration and S-configuration axial chiral enantiomers of lesinurad.
[0105] Enrichment and collection of peak components with a retention time of t=3.72min to obtain amorphous lesinurad whose axial chirality is S-...
Embodiment 3
[0115] The HEK293 cell line stably transfected with URAT1 (uric acid transporter) gene was used to determine the inhibitory activity of compounds on uric acid transporter.
[0116] Experimental Materials
[0117] (1) HEK293-pcDNA3.1-URAT1-4, HEK293-pcDNA3.1-5 cells: the HEK293 cell line stably transfected with URAT1 gene, the HEK293 cell line transfected with empty expression vector pcDNA3.1. The two cell lines were constructed by Shanghai WuXi AppTec New Drug Development Co., Ltd.
[0118] (2) Compounds to be tested: R-lesinurad (Formula II), S-lesinurad (Formula III), and lesinurad (Formula I), prepared with 100% DMSO solution to prepare 250 mM mother liquor for temporary storage in a nitrogen cabinet.
[0119] See Table 1 for other reagents.
[0120] Table 1. List of Reagents
[0121]
[0122]
[0123] experimental method
[0124] Cell treatment: add the cells HEK293-pcDNA3.1-URAT1-4 and HEK293-pcDNA3.1-5 to the 24-well plate coated with rat tail collagen, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com