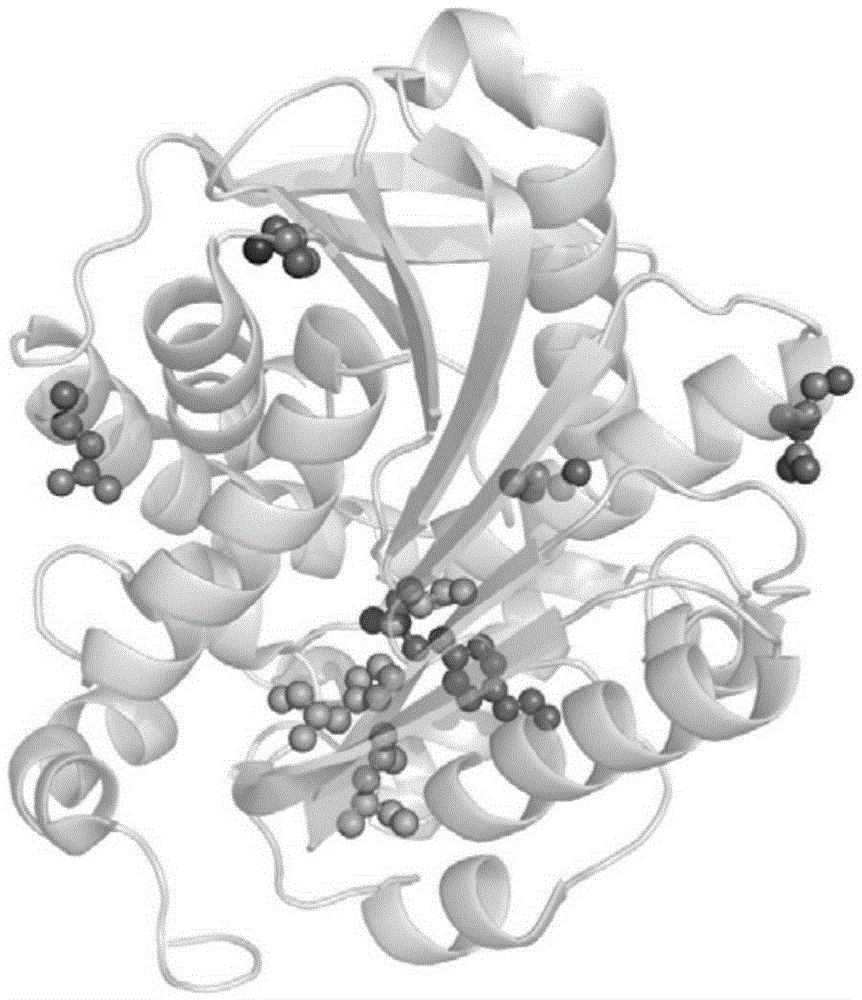
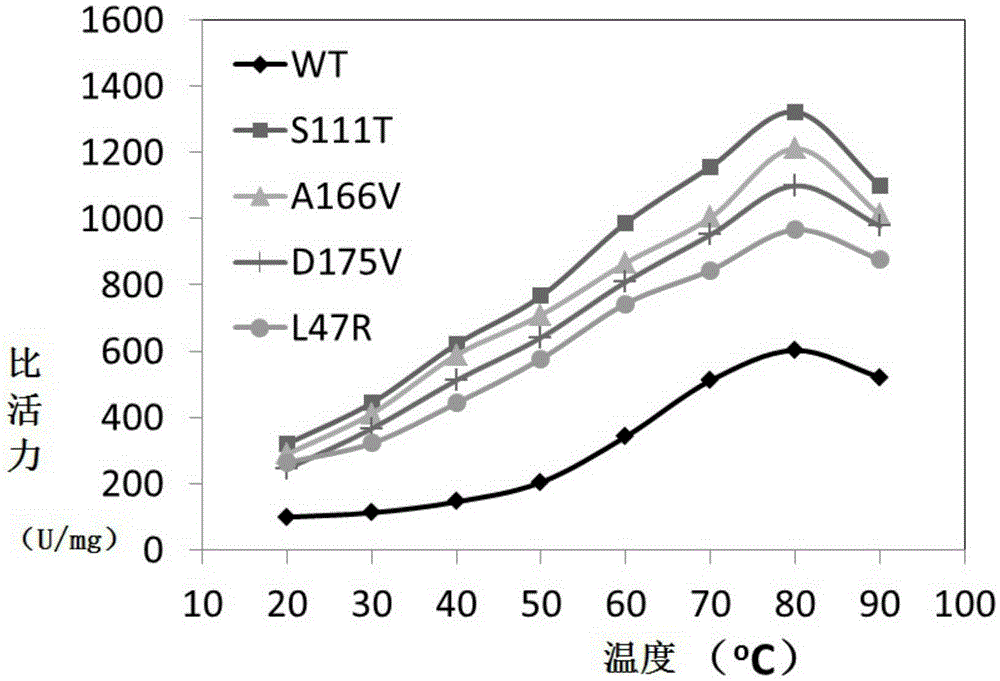
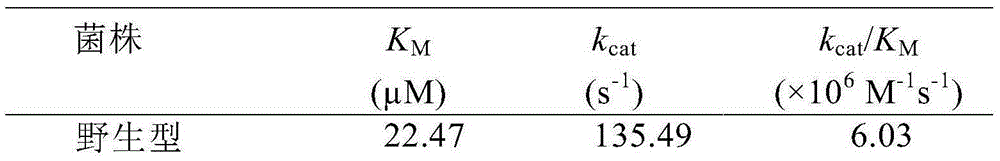
Thermophilic esterase AFEST mutant and screening method and application thereof
A technology for mutants and thermophilic esters, which is applied in the field of thermophilic esterase AFEST mutants and its screening, can solve the problems of directed evolution screening that cannot obtain good results, low positive rate, time-consuming and laborious, etc., to shorten the screening cycle and express The effect of high level and shortened screening efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Cloning of embodiment 1 wild-type esterase AFEST gene
[0041] The wild-type esterase AFEST gene was synthesized by Beijing Huada Gene Company, and its sequence is SEQ ID NO.1. Through the upstream primer SEQIDNO.3: 5'-CCGCGCGGCAGC catATG CTTGATATGCCAATC-3' (underlined base is restriction endonuclease NdeI recognition site) and downstream primer SEQIDNO.4: 5'-GAGCTCGAATTC ggatcc CTAGTCGAACACAAGAAGAG-3' (the underlined base is the restriction endonuclease BamHI recognition site) amplifies the target gene. The PCR reaction uses Takara's PrimeSTARMax polymerase. The PCR reaction conditions are: 98°C for 2min, then 98°C for 10sec, 55°C 15sec at ℃, 10sec at 72℃, a total of 30 cycles; the last 10min at 72℃. After the reaction, the PCR amplification product was detected by 1% agarose gel electrophoresis, and a band with a size of 1 kb was obtained, which was consistent with the expected result. DpnI digests the template, recovers, purifies the target fragment, performs do...
Embodiment 2
[0042] Expression, purification and activity determination of embodiment 2AFEST
[0043] The engineered bacteria in the glycerol tube were inoculated into a 4 mL LB medium test tube containing 100 μg / mL Kan at a volume ratio of 1%, and cultured at 37° C. and 220 rpm for 12 hours. Transfer the 4mL bacterial liquid to a 1LLB medium shake flask containing 50μg / mLKan, culture at 37°C 220rpm for about 2.5h, make the OD600 reach about 0.8, add 0.1mMIPTG inducer, and induce culture at 25°C 200rpm for 12-16h. The Escherichia coli cell suspension harvested after fermentation is ultrasonically disrupted, and after one-step Ni-NTA affinity chromatography treatment, the target protein with a purity of more than 95% can be obtained. For the determination of esterase AFEST activity, refer to the literature ArchBiochemBiophys.2000, 373(1): 182-92.
Embodiment 3
[0044] The construction of the large-capacity random mutation library of embodiment 3 esterase AFEST
[0045] The mutation library of AFEST is constructed by error-prone PCR (ep-PCR), wherein the mutation rate is achieved by adjusting the concentration of manganese ions in the PCR system. The error-prone PCR system is: DreamTaq TM (0.05U / μL) and its buffer (Takara), dATP(250μM), dGTP(250μM), dCTP(1050μM), dTTP(1050μM), AFESTupper(0.4μM), AFESTlower(0.4μM), AFEST-pET-28a Plasmid (0.2ng / μL), manganese chloride (0.2-0.8mM). Wherein AFESTupper sequence SEQIDNO.3 is 5'-CCGCGCGGCAGC catATG CTTGATATGCCAATC-3', AFESTlower sequence SEQ ID NO.4 is 5'-GAGCTCGAATTC ggatcc CTAGTCGAACACAAGAAGAG-3'. Aliquot the PCR system into 25 μL tubes for error-prone PCR (95°C for 3min, 1cycle; 95°C for 15s / 55°C for 30s / 72°C for 1min, 30cycles; 72°C for 5min, 1cycle). The purified target fragment was double digested with NdeI and BamHIII, then cloned into the vector pET28a with T4 ligase, and then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com