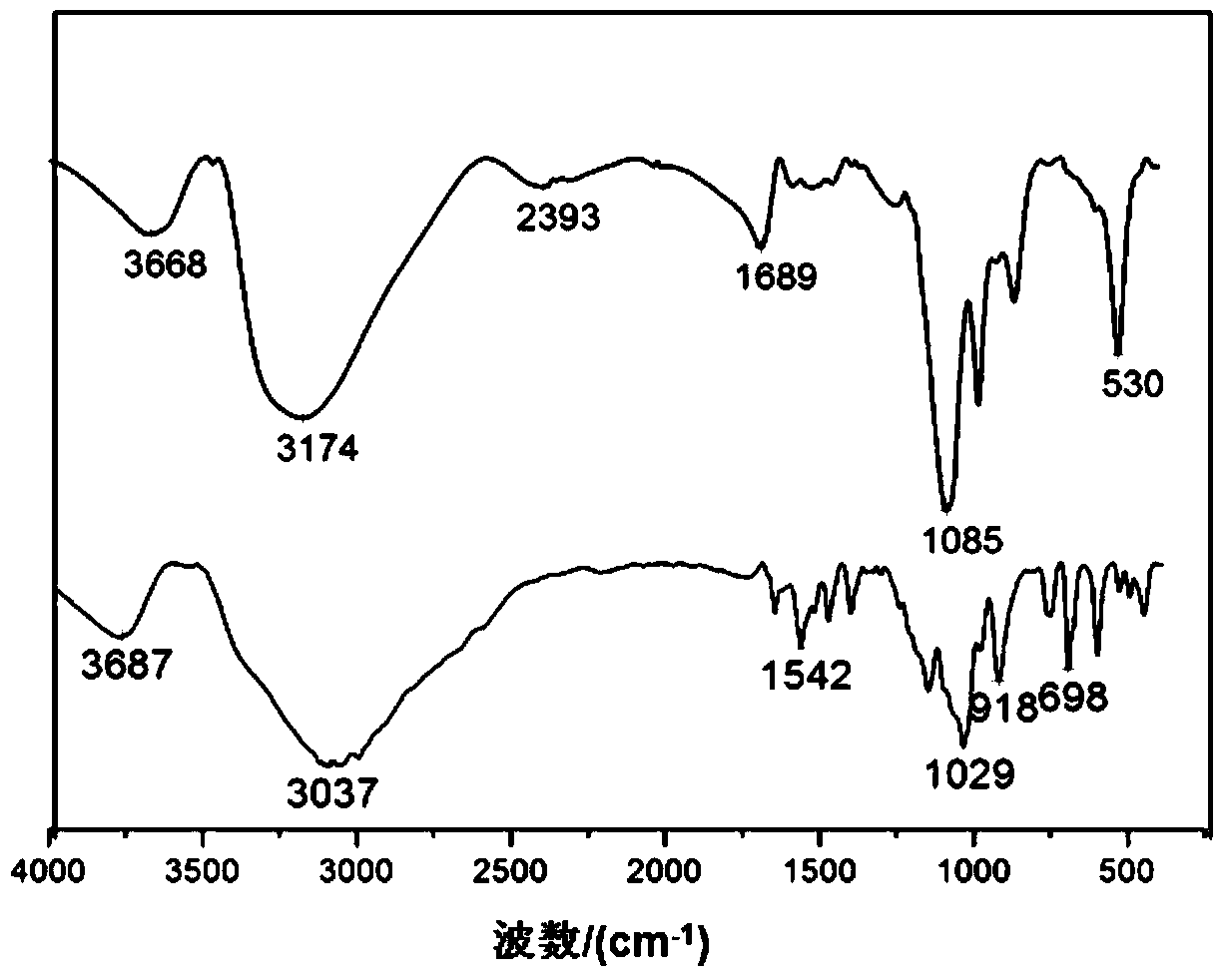
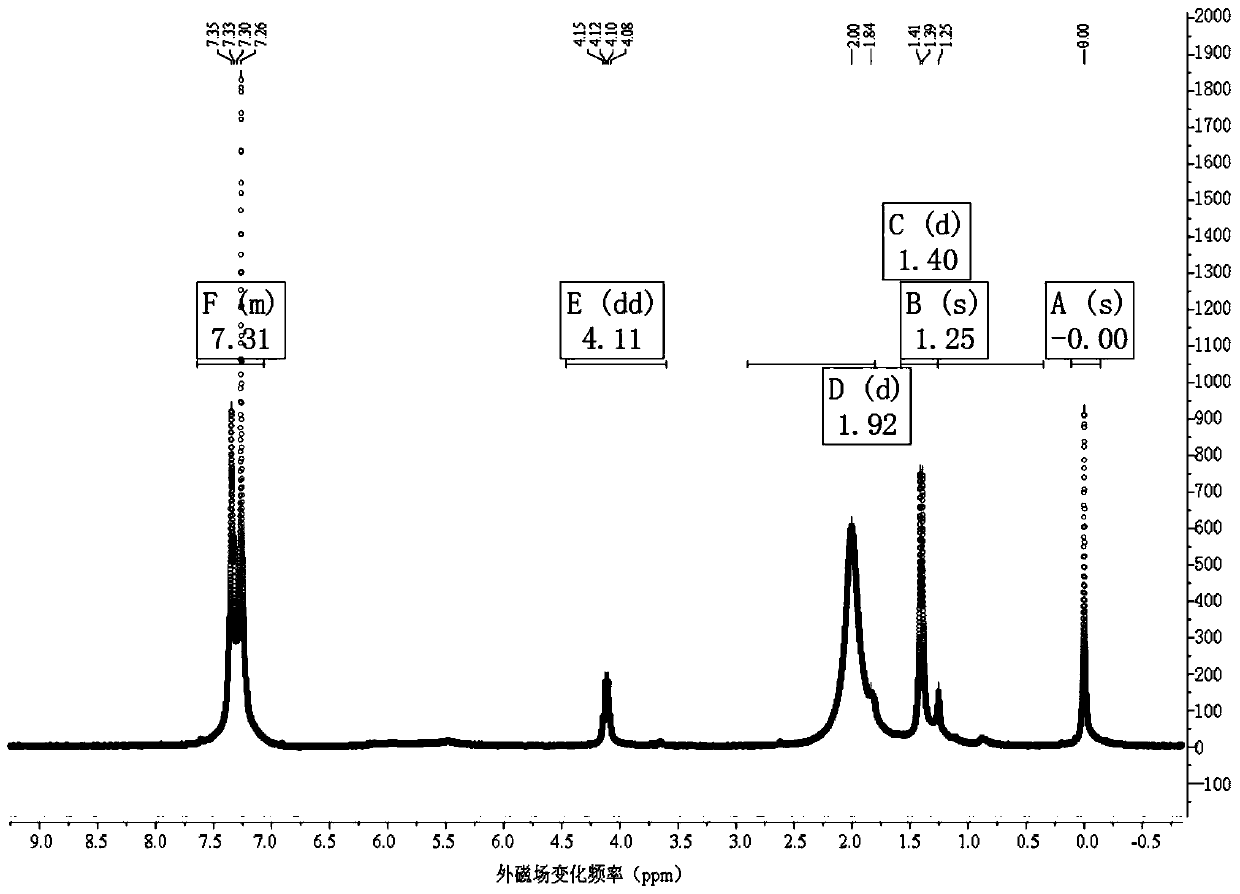
Chloroperoxidase one-step catalytic method for synthesizing chiral drug fosfomycin preparation
A technology of chloroperoxidase and chiral drugs, which is applied in the field of synthesizing chiral drug fosfomycin preparations by one-step catalysis of chloroperoxidase, which can solve the problems of single-use environment, unfavorable industrial production, and large water pollution, etc. problem, to achieve the effect of high yield, heavy workload and high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Take 1 mmol of industrial grade cis-propenyl phosphoric acid with a mass percentage of 70% and dissolve it in 2 mL of pH=4 phosphate buffer, add 10 mmol·L -1 The sodium carbonate is adjusted to pH = 5.5, and the initial aqueous solution of the substrate is prepared. Then add the oxidant and the chloroperoxidase solution prepared with the phosphate buffer solution of pH=5 to the initial aqueous solution of the substrate, wherein the mass ratio of cis-propenyl phosphate, oxidant and chloroperoxidase is 2:7 : 5×10 -5 After mixing evenly, continue to shake and react at room temperature for 0.5h. After the reaction is completed, the reaction solution is heated and evaporated to obtain a sample, and the sample is cooled to crystallize and vacuum-dried to obtain the chiral drug fosfomycin sodium.
[0038] In order to investigate the effect of adding an oxidant on the yield, according to the required amount, add hydrogen peroxide H prepared by twice ultrafiltered distilled wat...
Embodiment 2
[0040] Take 1mmol of industrial grade cis-propenyl phosphoric acid with a mass percentage of 70% and dissolve it in 1mL of phosphate buffer solution with pH=4, add 3mmol·L -1 The sodium hydroxide is adjusted to pH=5, and the initial aqueous solution of the substrate is prepared. In order to investigate the effect of different amounts of immobilized CPO on the yield, 3.4 mmol of H 2 o 2 Oxidant and the CPO diluted with the phosphate buffer solution of pH=5, the dosage of CPO is 0, 0.0167, 0.025, 0.0333, 0.047, 0.0667, 0.075, 0.0833 and 0.1 μmol; then continue shaking reaction at room temperature for 1.5h, the reaction After completion, the reaction liquid is heated and evaporated to obtain a sample, which is then crystallized by cooling and vacuum-dried to obtain the chiral drug fosfomycin sodium.
[0041] When no immobilized CPO was added, the conversion rate of the intermediate product of cis-propenyl phosphate was very low, almost zero, indicating that the 2 o 2 When thi...
Embodiment 3
[0043] Take 1.5mmol of industrial-grade, 70% by mass cis-propenyl phosphoric acid and dissolve it in 2.5mL of phosphate buffer with pH=5.5, add 10mmol·L -1 The sodium carbonate was adjusted to pH=6, and the initial aqueous solution of the substrate was prepared.
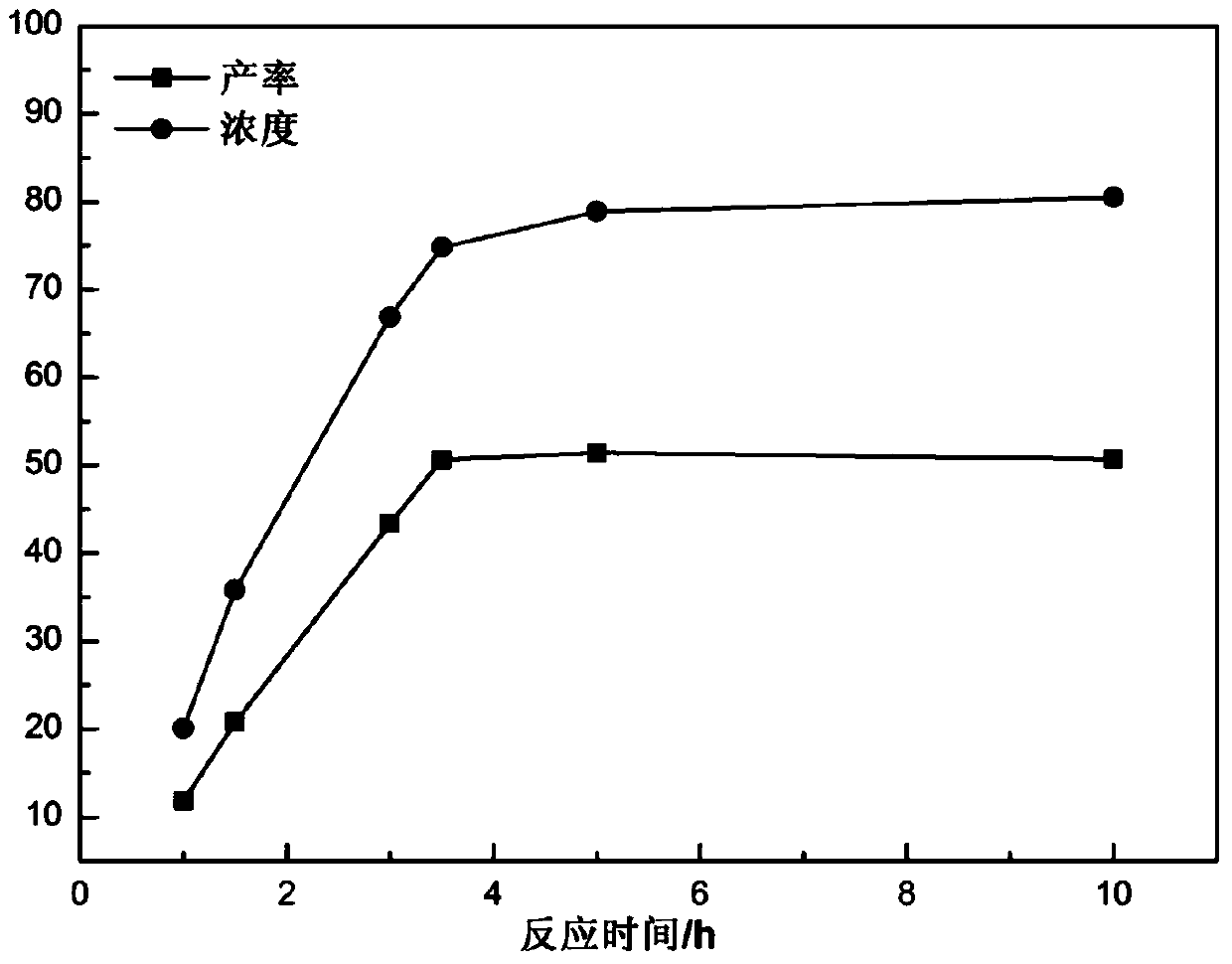
[0044] Add H to the initial aqueous solution of the substrate 2 o 2 Oxidant and CPO solution prepared with phosphate buffer solution of pH=5, wherein the mass ratio of cis-propenyl phosphate, oxidant and chloroperoxidase is 2:6:5×10 -5 , after mixing evenly, continue shaking and reacting at room temperature for 1-10 hours. After the reaction is completed, the reaction solution is heated and evaporated to obtain a sample, and the sample is cooled to crystallize and vacuum-dried to obtain the chiral drug fosfomycin sodium. The yield of fosfomycin sodium was measured at regular intervals.
[0045] see figure 1 The influence of reaction time on the asymmetric epoxidation reaction of cis-acryl phosphate catalyzed by C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com