Erlotinib hydrochloride tablet and preparation method thereof
A technology for erlotinib hydrochloride tablets and erlotinib hydrochloride, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., and can solve the risk of biological inequivalence. problem, to achieve the effect of easy sieving, not easy to stick and punch, and the particle size of raw materials increases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation process of the pharmaceutical composition of the present invention is as follows: the raw materials and auxiliary materials are all passed through a 60-mesh sieve, mixed uniformly in an equal-volume incremental manner, then wet-granulated with an aqueous solution of sodium lauryl sulfate, dried, and added with magnesium stearate. Blending, tableting, coating. The dosage range is as follows:
[0036] Process steps
final parameter
Parameter control recommended range
preferred range range
Raw material sieve mesh
60 mesh
60~100 mesh
60~80 mesh
Concentration of sodium lauryl sulfate aqueous solution (w / w)
0.5%
0.4%~0.6%
0.4%~0.5%
1~2%
1~5%
1~3%
Tablet hardness
50~70N
40~100N
50~80N
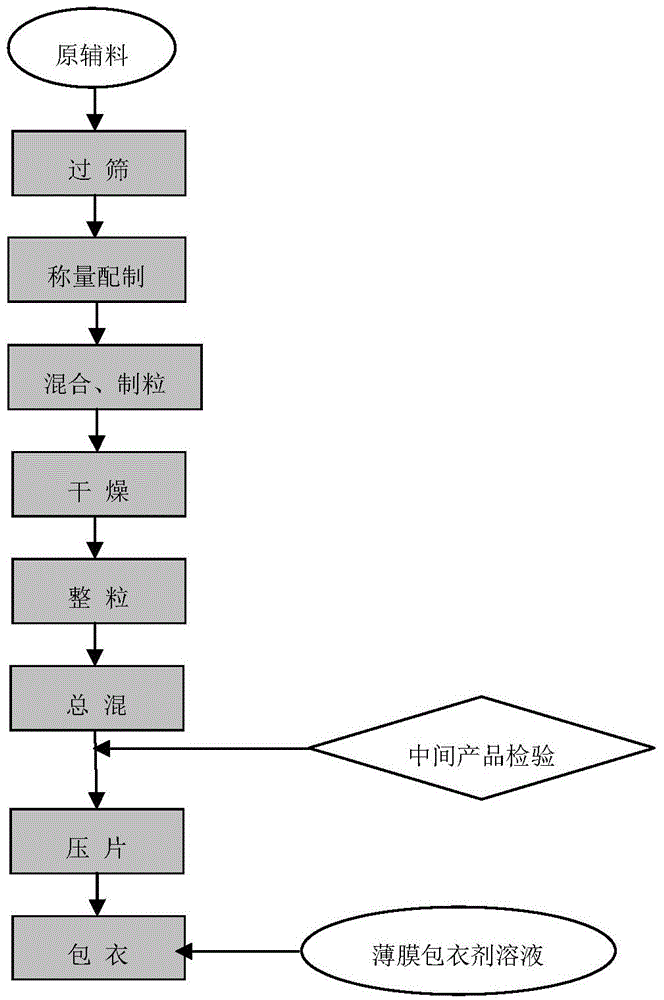
[0037] The process flow diagram of the present invention is as figure 1 Shown:
[0038] Process flow of the present invention is described as follows: ...
experiment example 1
[0050] Experimental Example 1: Erlotinib Hydrochloride Tablets Containing Raw Materials of Different Particle Sizes
[0051]
[0052] Preparation method: mix erlotinib hydrochloride (passed through a corresponding mesh sieve) and silicon dioxide, lactose, microcrystalline cellulose, sodium carboxymethyl starch (internal addition), and mix with sodium lauryl sulfate aqueous solution Wet granulation, drying, adding sodium carboxymethyl starch (additional), mixing evenly, adding magnesium stearate, mixing evenly and pressing into tablets.
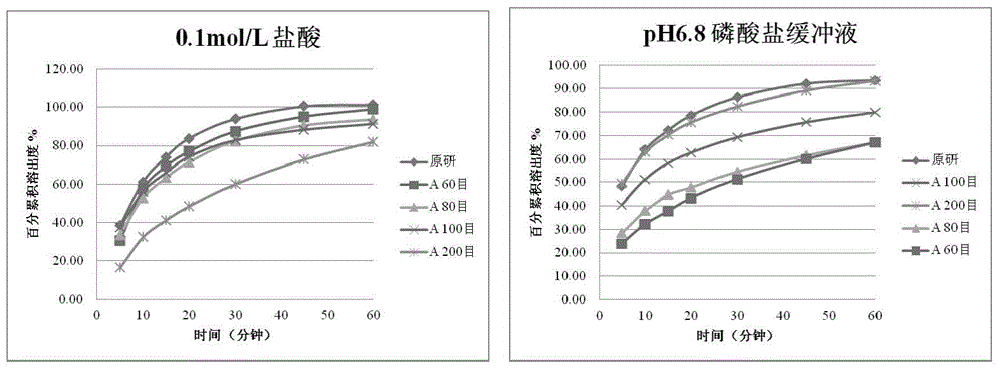
[0053] The results are shown in Table-1 and figure 2 .
[0054] Table-1. Particle size screening results of raw materials
[0055]
[0056] From the above results, it can be seen that the change of the particle size of the raw material has no obvious impact on the disintegration time of the tablet core, but has a significant impact on the particle properties after disintegration. With the decrease of the particle size of the raw mater...
Embodiment 2
[0058] Embodiment 2: sodium carboxymethyl starch dosage screening
[0059]
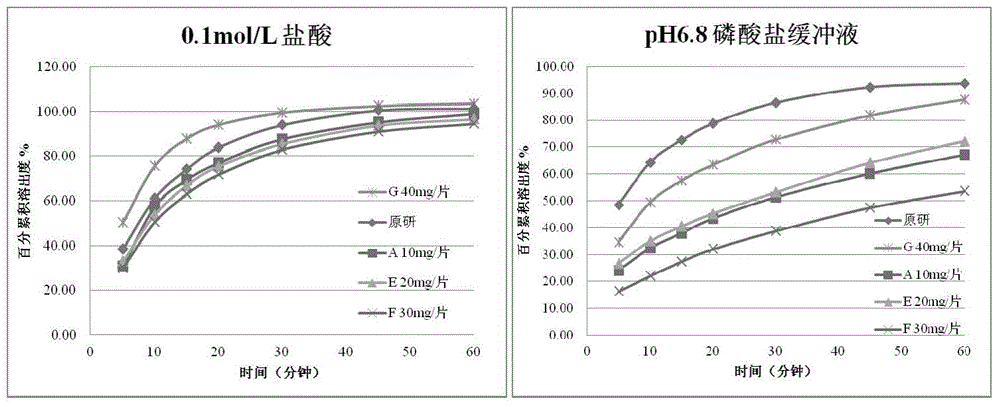
[0060] Preparation method: with embodiment 1. The results are shown in Table-2 and image 3 .
[0061] Table-2 Screening Results of Sodium Carboxymethyl Starch Dosage (n=3)
[0062]
[0063] From the above results, it can be seen that on the basis of prescription A, the dosage of disintegrant sodium carboxymethyl starch increased from 10mg / tablet to 30mg / tablet, the disintegration time of the tablet increased, and the disintegration behavior also changed significantly. It shows the phenomenon of swelling first and then disintegrating; within this range, the dissolution curve in 0.1mol / L hydrochloric acid has no significant change, but the dissolution curve in pH6.8 phosphate buffer solution decreases (prescription F: 30mg / tablet). When the internal disintegrant was increased to 40mg / tablet, the disintegration time of the tablet did not slow down further, and the dissolution in both media was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com