A preparing method of a vanadium phosphorus oxide catalyst for n-butane oxidation to produce maleic anhydride
An anhydride vanadium phosphorus oxygen catalyst, catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, organic chemistry, etc., to achieve the best catalytic performance, high maleic anhydride selectivity, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] (2) Preparation of vanadium phosphorus oxygen catalyst
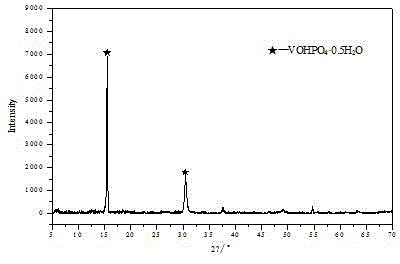
[0050] The vanadium phosphorus oxide obtained in step (1) is first shaped, and the shape of the obtained vanadium phosphorus oxygen catalyst can be a tablet, a spherical shape, an extruded rod, etc., and the phase of the catalyst precursor is (VOHPO 4 0.5H 2 o).
[0051] (3) Activation of vanadium phosphorus oxygen catalyst
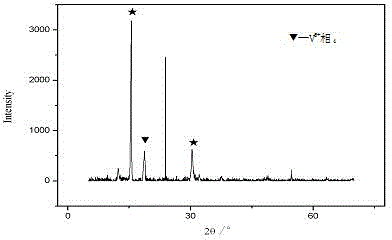
[0052] Take 86g of the Raschig ring-shaped catalyst particles obtained in step (2), impregnate them into 180g of 2.5% tert-butyl hydroperoxide tert-butanol solution, keep the impregnation time for 1-2 hours, filter the catalyst after the impregnation, and put the wet catalyst Dry in a closed drying oven at 100~150°C, recover the evaporated tert-butanol solvent, put the dried catalyst into a tubular reactor, and in an inert gas or nitrogen atmosphere, at a gas volume space velocity of 100~1500h -1 , the heating rate is 1-5°C / min, the activation temperature is raised to 425°C for roasting tr...
Embodiment 1
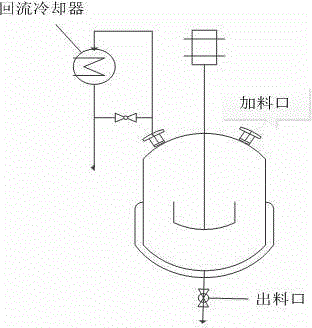
[0057] exist figure 1 In the shown reaction kettle with stirring device and reflux condensing device, add 649mL of isobutanol and benzyl alcohol mixture, the volume ratio of isobutanol / benzyl alcohol is 10:1, 29.53g of vanadium pentoxide, additive hexahydrate Ferric nitrate 0.3g, additive zirconium nitrate 0.5g, start stirring, increase the reaction temperature and keep it at 100±2°C, carry out reflux reaction, keep reflux time for 4 hours, then add 34.98g of phosphoric acid with a concentration of 100%, phosphorus / vanadium The molar ratio was 1.1, the reflux was continued for 4 hours, and the reaction ended. After the reaction solution was cooled to room temperature, it was vacuum filtered, and the filter cake was rinsed with a small amount of isobutanol three times, then the filter cake was placed in an enamel tray and dried naturally at room temperature, dried in an oven at 100°C for 8 hours, and finally dried in a muffle furnace. Calcined at 250°C for 5 hours to obtain a ...
Embodiment 2
[0063] exist figure 1 In the shown reaction kettle with stirring device and reflux condensing device, add 649mL of isobutanol and benzyl alcohol mixture, the volume ratio of isobutanol / benzyl alcohol is 10:1, 29.53g of vanadium pentoxide, additive hexahydrate Ferric nitrate 0.3g, additive zirconium nitrate 0.5g, start stirring, increase the reaction temperature and keep it at 100±2°C, carry out reflux reaction, keep reflux time for 4 hours, then add 34.98g of phosphoric acid with a concentration of 100%, phosphorus / vanadium The molar ratio was 1.1, the reflux was continued for 4 hours, and the reaction ended. After the reaction solution was cooled to room temperature, it was vacuum filtered, and the filter cake was rinsed with a small amount of isobutanol three times, then the filter cake was placed in an enamel tray and dried naturally at room temperature, dried in an oven at 100°C for 8 hours, and finally dried in a muffle furnace. Calcined at 250°C for 5 hours to obtain a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com