Synthesizing method for 5E-decene-1-alcohol and acetic acid 5E-decenoate
A synthetic method and technology of decene, applied in the preparation of carboxylic acid esters, chemical instruments and methods, and preparation of carbon-based compounds, etc., can solve problems such as high cost, difficult industrial production, and complicated steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
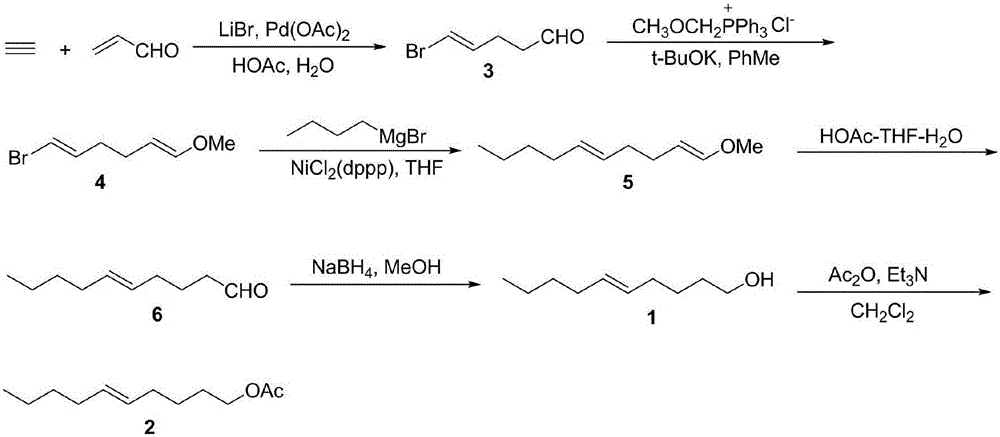
[0033] The invention provides a kind of synthetic method of 5E-decen-1-ol, comprising the steps of:
[0034] (1) In a solution system, acrolein, lithium bromide and acetylene are subjected to a coupling reaction in the presence of a palladium catalyst to obtain 5-bromo-4E-pentenal;
[0035] (2) Under inert gas protection conditions, methoxymethyltriphenylphosphine chloride, basic substance, 5-bromo-4E-pentenal and anhydrous organic solvent are mixed for reaction to obtain 1E, 5E- 1-bromo-6-methoxyhexadiene;
[0036] (3) Under inert gas protection conditions, the catalyst, 1E, 5E-1-bromo-6-methoxyhexadiene, and n-butylmagnesium bromide are dissolved in anhydrous organic solvent and reacted to obtain 1E, 5E- 1-Methoxydecadiene;
[0037] (4) dissolving the 1E,5E-1-methoxydecadiene obtained in the step (3) in a mixed solvent of acetic acid, tetrahydrofuran and water, and undergoing a hydrolysis reaction to obtain 5E-decenal;
[0038] (5) Mix 5E-decenal, a reducing agent, sodium...
Embodiment 1
[0077] Dissolve 2.5 mol of acrolein, 3 mol of lithium bromide and 2.5 mmol of palladium acetate in a mixed solvent of acetic acid and water, pass through acetylene scrubbed with sulfuric acid, stir and react at room temperature for 48 hours, add saturated sodium carbonate solution to neutralize properties, separated the organic layer, extracted the aqueous layer with 3×200mL dichloromethane, combined the organic layers and washed with water, and then washed with anhydrous Na 2 SO 4 The organic layer was dried, and the solvent was evaporated to obtain 350 g of 5-bromo-4E-pentenal, with a yield of 86%;
[0078] Under the protection of nitrogen and in an ice bath, dissolve 1.5 mol of methoxymethyltriphenylphosphine chloride in anhydrous tetrahydrofuran, add 1.5 mol of potassium tert-butoxide in 3 times, and stir for 1 hour to obtain a red solution. -Bromo-4E-pentenal 1mol 1L anhydrous tetrahydrofuran solution was added dropwise to the reaction mixture, stirred and reacted for 12...
Embodiment 2
[0084] Dissolve 3 mol of acrolein, 3.6 mol of lithium bromide and 3 mmol of palladium chloride in trifluoroacetic acid, pass through acetylene scrubbed with sulfuric acid, stir and react at room temperature for 36 hours, add saturated sodium carbonate solution to neutralize until neutral, separate The organic layer and the aqueous layer were extracted with 3×200mL ether, the combined organic layers were washed with water, and then washed with anhydrous Na 2 SO 4 The organic layer was dried, and the solvent was evaporated to obtain 425 g of 5-bromo-4E-pentenal, with a yield of 87%;
[0085] Under the protection of nitrogen and in an ice bath, dissolve 2 mol of methoxymethyltriphenylphosphine chloride in toluene, add 2.1 mol of sodium hydride in 3 portions, stir for 1 hour to obtain a red solution, and 5-bromo-4E- 1L toluene solution of 2 mol of pentenal was added dropwise to the reaction mixture, stirred and reacted for 12 hours, 1L of water was added, the water layer was sepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com