Preparation method of nitrogen-enriched porous carbon desulfurizer with superhigh specific surface area
A technology of ultra-high specific surface area, porous carbon, applied in separation methods, chemical instruments and methods, catalysts for physical/chemical processes, etc., can solve the problems of cumbersome preparation process, low carbon yield, high energy consumption, and achieve broad application prospects , The effect of simple process and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
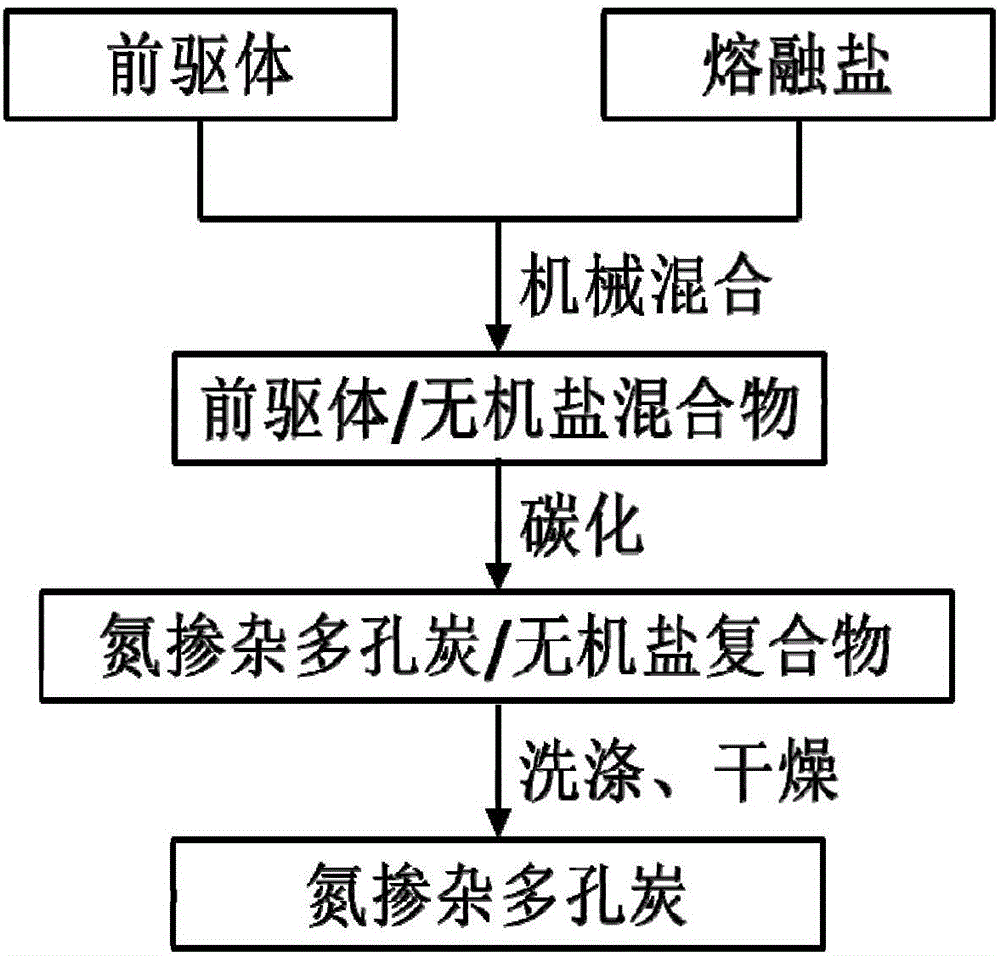
[0040] figure 1 It is a schematic diagram of the preparation process of nitrogen-doped porous carbon in the present invention, and the specific preparation process is as follows:
[0041] (1) Combine small molecule precursors (adenine or glycine or glucose and melamine) with molten salt (KCl-ZnCl 2 or NaCl-ZnCl 2 ) for mechanical mixing, the mechanical mixing methods used include grinding, ball milling or a combination of grinding and ball milling;
[0042] (2) Place the precursor and molten salt mixture in an inert atmosphere for carbonization treatment, and the inert protective gas used is selected from one or more mixtures of nitrogen, argon, helium and other gases;
[0043] (3) The carbonized product is washed with acid and deionized water, and the acid used is selected from one or more mixtures of inorganic acids such as hydrochloric acid, sulfuric acid, nitric acid, and phosphoric acid.
Embodiment 1
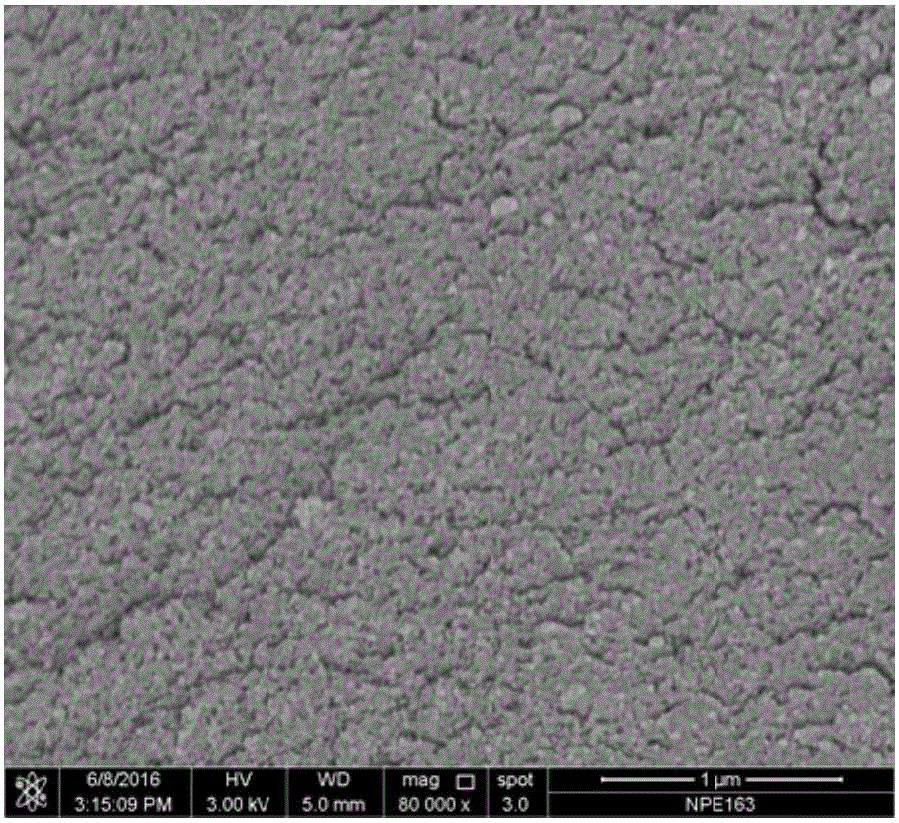
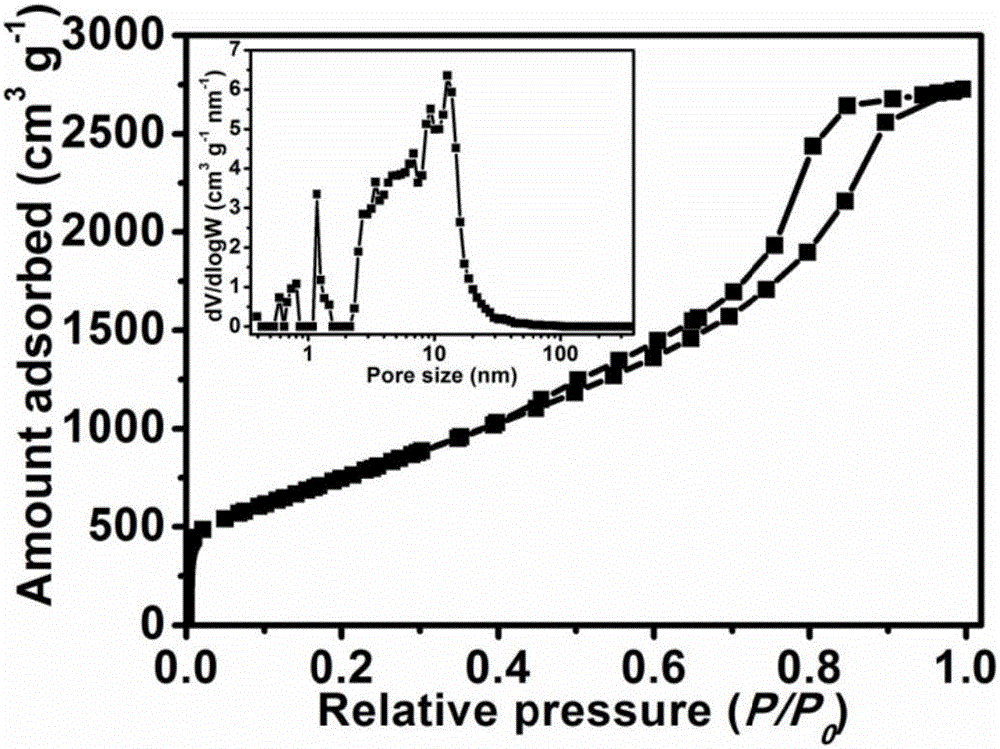
[0046] 1.0g adenine, 1.90g KCl and 8.10g ZnCl 2 (KCl in KCl-ZnCl 2 The mole fraction in is 0.3) mixing and grinding evenly. The mixture was placed in a quartz tube, heated to 800 °C in a nitrogen atmosphere and kept at a constant temperature for 1 h for carbonization. Cool to room temperature. Product in 1mol L -1 Stir in hydrochloric acid solution for 12 hours, filter with suction, wash with deionized water, and dry to obtain a nitrogen-doped porous carbon material (denoted as NPC-A-K / Zn0.3-800). The elemental analysis results show that the nitrogen content in the nitrogen-doped porous carbon is 20.79wt.%. figure 2 Shown is a scanning electron microscope picture of the NPC-A-K / Zn 0.3-800 sample, from which it can be seen that the sample is composed of very small nanoparticles. image 3 It is the nitrogen adsorption-desorption curve and pore size distribution curve of the sample. The nitrogen adsorption test results show that the sample presents micropore-mesopore charac...
Embodiment 2
[0048] 1.0g adenine, 3.54g KCl and 6.46g ZnCl 2 (KCl in KCl-ZnCl 2 The mole fraction in is 0.5) mix and grind evenly. The mixture was placed in a quartz tube, heated to 800 °C in a nitrogen atmosphere and kept at a constant temperature for 1 h for carbonization. Cool to room temperature, the product is in 1mol L -1 Stir in hydrochloric acid solution for 12 hours, filter with suction, wash with deionized water, and dry to obtain a nitrogen-doped porous carbon material (denoted as NPC-A-K / Zn 0.5-800). Elemental analysis results showed that the nitrogen content was 21.94wt.%. Figure 5 Shown is a scanning electron microscope picture of the NPC-A-K / Zn 0.5-800 sample, from which it can be seen that the sample is composed of aggregated carbon spheres. Image 6 It is the nitrogen adsorption-desorption curve and pore size distribution curve of the sample. The nitrogen adsorption test results show that the sample presents micropore-mesopore characteristics, and the specific surface...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com