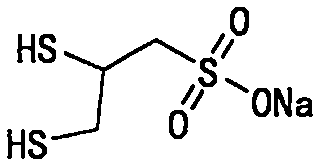
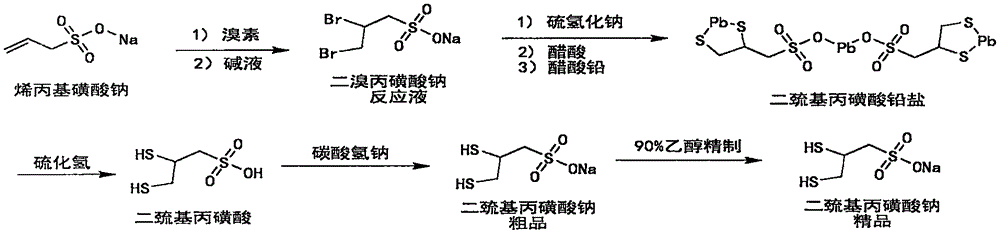
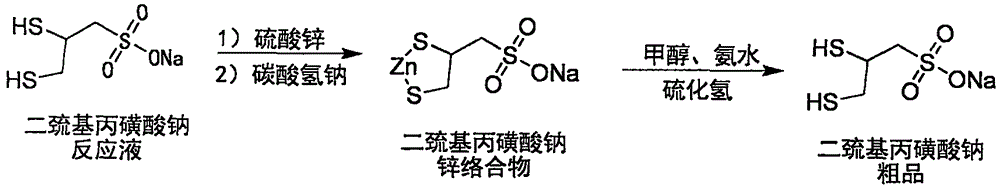
Sodium dimercaptopropansulfonate preparation method
A technology of sodium dimercaptopropanesulfonate and sodium dimercaptopropanesulfonate, which is applied in the field of chemical pharmaceuticals, can solve the problems of low solubility, difficult stirring, and environmental pollution, and achieve the effect of increasing the content and avoiding strong acidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0062] The preparation of embodiment 1 sodium dimercaptopropanesulfonate
[0063] Step a: Preparation of Sodium Zinc Dimercaptopropanesulfonate Complex
[0064] Into a 100L glass reactor, add 40.0kg of purified water, start stirring (medium speed, 100-120rpm), and add 10.36kg (content 93%, 66.85mol, 1.0 equivalent) sodium allyl sulfonate, 1.30 kg (12.63mol, 0.19 equivalent) of sodium bromide, and turn on the low-temperature cooling circulation pump for cooling. Slowly add 10.0 kg of bromine (62.58 mol, 0.936 eq) dropwise at an internal temperature of 5±3°C, and the dropwise addition is completed in about 4 hours. After the dropwise addition, keep stirring for 10 minutes. 20% sodium hydroxide was slowly added dropwise to adjust the pH of the reaction solution to 6-7, and a total of 1.30 kg (32.50 mol) of sodium hydroxide was consumed. Add 7.5kg (content 70%, 94.37mol, 1.41 equivalent) of sodium hydrosulfide solid, control the water bath at 35±5°C, keep warm and stir for 3 hou...
Embodiment 2
[0073] Embodiment 2 (preparation of sodium dimercaptopropanesulfonate zinc complex, without additives)
[0074] In this embodiment, no auxiliary agent is added during the bromination reaction, and other steps and reaction conditions are the same as step a and step b of embodiment 1.
[0075] Result: Consumption of sodium hydroxide 1.60kg (40.05mol), dimercaptopropanesulfonate sodium zinc complex boutique: 3.86kg (14.11mol), liquid phase purity 90.9%, 95.2% after deducting zinc sulfide, calculated as zinc acetate, Yield 30.9%.
Embodiment 3
[0076] Embodiment 3 (preparation of dimercaptopropanesulfonate sodium zinc complex, auxiliary agent sodium bromide consumption is reduced to 0.1 equivalent)
[0077] In this example, the additive sodium bromide was reduced from 1.30kg (12.63mol, 0.19equivalent) to 684.2g (6.65mol, 0.10equivalent), and other steps and reaction conditions were the same as step a and step b of Example 1.
[0078] Result: Consumption of sodium hydroxide 1.44kg (36.02mol), dimercaptopropanesulfonate sodium zinc complex boutique: 4.75kg (17.36mol), liquid phase purity 91.6%, 96.3% after deducting zinc sulfide, calculated with zinc acetate, Yield 38.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com