Donor-acceptor type fluorenyl nanometer grid material, preparation method and application thereof
A nano-lattice, donor-acceptor-type technology, which is applied in the field of donor-acceptor-type fluorenyl nano-lattice materials and its preparation, can solve the problems of low reactivity of acceptor fragments, low ring-forming yield of cyclic molecules, and difficulty in separation. , achieve the effects of reducing film solvent dependence, modular synthesis, and high scalability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
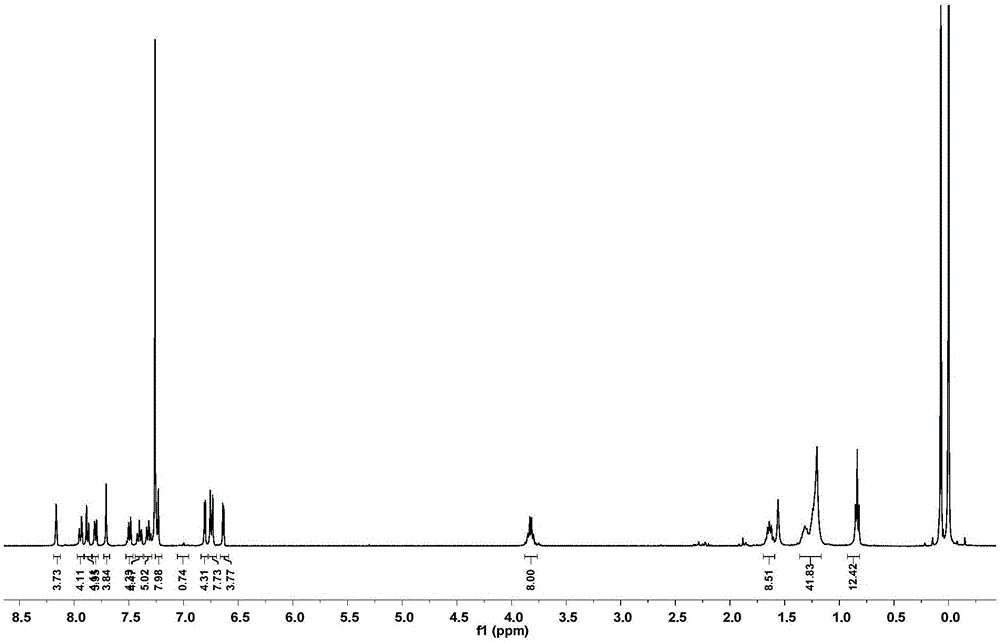
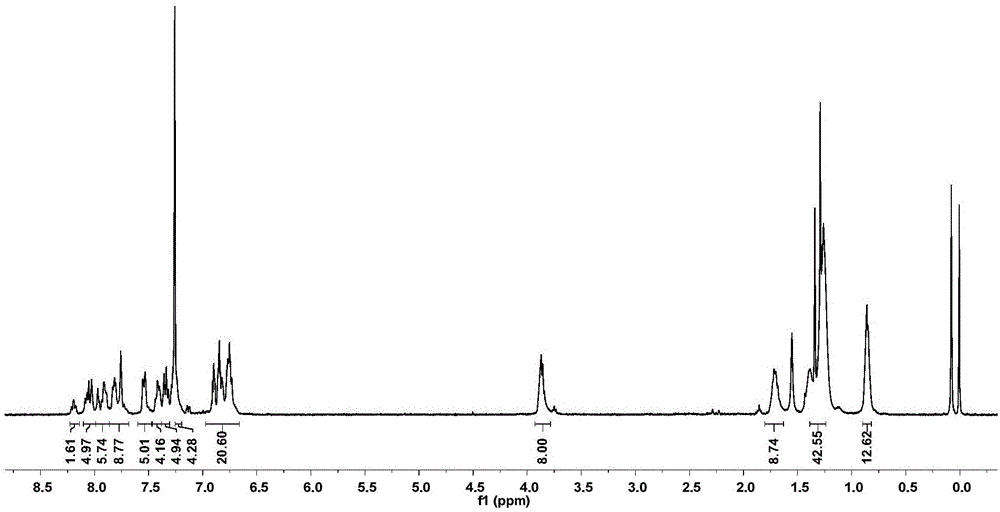
[0061] When W is C; Ar 1 、Ar 2 For p-bromooctyloxybenzene, Ar 3 For dithiophene or triple thiophene, Ar 4 It is benzothiadiazole, G1 and G2 are both hydrogen, and the lattice molecular structures are as follows:
[0062]
[0063] The reaction scheme is as follows:
[0064]
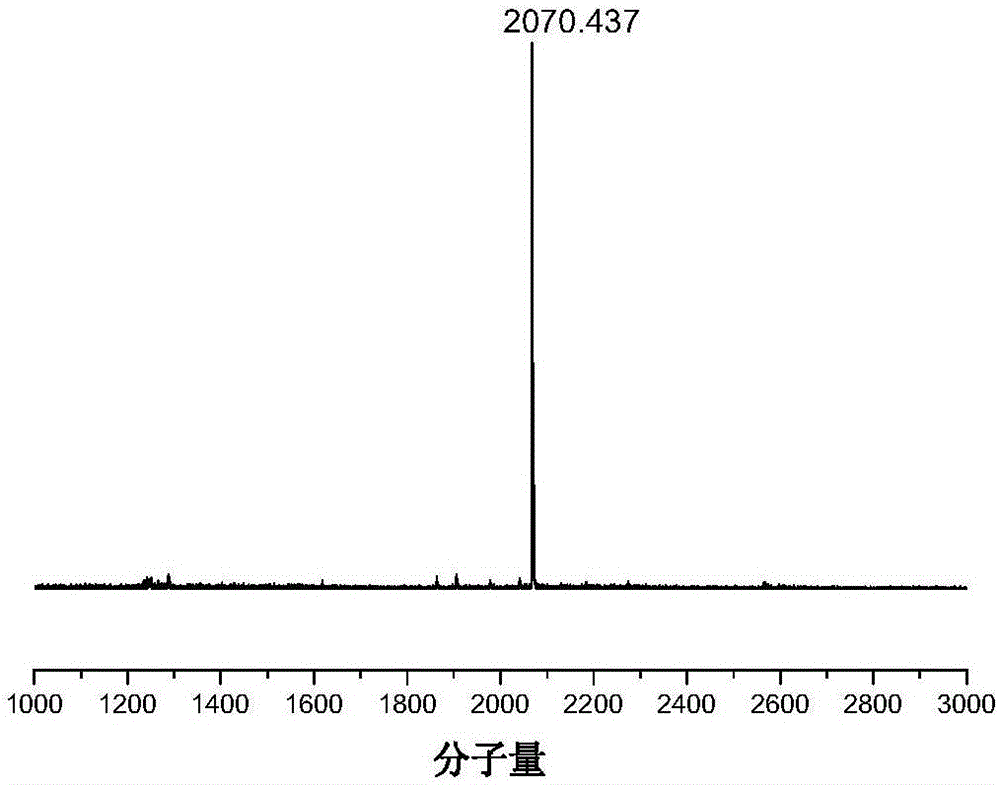
[0065] The specific preparation method is as follows: dithiophene and tertiary thiophene are obtained by coupling thiophene monomers under the catalysis of Pd / C; bisbromobenzobithiazole is borated at high temperature to obtain compound 3; –Crafts reaction to obtain compound 5, compound 3, 4, 5 in Pd(PPh 3 ) 4 Catalyst, alkaline solution choose K 2 CO 3 Under Suzuki coupling under / KF conditions, the monosubstituted L-shaped precursor can be obtained efficiently. The L-shaped precursor is located in Et through its own double active site tertiary alcohol group and the 2-position of thiophene 2 O·BF 3 Catalyzed Friedel–Crafts reaction ring closure to obtain the acceptor-type fluorenyl lattice ...
Embodiment 2
[0077] Thermogravimetric analysis (TGA) was performed on a Shimadzu DTG-60H thermogravimetric analyzer with a heating scan rate of 10 °C / min and a nitrogen flow rate of 20 cm 3 / min. Differential scanning calorimetry (DSC) was carried out on a Shimadzu DSC-60A tester. The sample was first heated at a rate of 10°C / min to a state ten degrees lower than the decomposition temperature of the sample, and then, under liquid nitrogen conditions The temperature was lowered back to the starting temperature, and the temperature was scanned at a rate of 10°C / min for the second time. From the TGA experiment, the temperature (T d ) are 355°C and 320°C, respectively. DSC experiments showed no obvious glass transition temperature.
Embodiment 3
[0079] Prepare the nanogrids G1 and G2 into accurate 1 μM dichloromethane dilute solutions respectively, and flush with argon gas to remove oxygen. The absorption and emission spectra were measured by Shimadzu UV-3150 ultraviolet-visible spectrometer and RF-530XPC fluorescence spectrometer.
[0080] Such as Figure 5 , 6 As shown, the peaks of the ultraviolet absorption spectrum and fluorescence spectrum of the nano-lattice G1 in dichloromethane are 381nm and 530nm, respectively. The peaks of the ultraviolet absorption spectrum and fluorescence spectrum of nano-lattice G2 in dichloromethane are 408nm and 534nm, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com