Novel hapten for tebuconazole as well as synthetic method and application thereof
A synthetic method and hapten technology applied in the field of hapten to achieve the effect of simple synthetic method, high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] (2) Preparation of immunogen
[0050] Weigh tebuconazole hapten (4.1 mg, 0.01 mmoL) into N-N-dimethylformamide (DMF, 1 mL), stir to dissolve, add 1-ethyl-carbodiimide hydrochloride ( EDC, 4.5 mg) and N-hydroxysuccinimide (NHS, 4.4 mg), react at room temperature for 6 hours to obtain liquid A.
[0051] Take hemocyanin (KLH, 20.92 mg / mL, 0.3 mL) and add it to borate buffer solution (0.2 mol / L, 3.5 mL, pH 8.8), stir and mix to obtain solution B. Add solution A to solution B dropwise under ice-cooling, and stir the reaction at 4°C for 8-10 hours.
[0052] The reaction solution was dialyzed against phosphate buffer solution (0.01mol / L, pH 7.2) for 3 days, and the dialysate was changed every 6 hours for 8 times. The obtained tebuconazole immunogen is subpackaged, frozen and stored for future use.
[0053] (3) Preparation of coating agent
[0054] Weigh tebuconazole hapten (4.1 mg, 0.01 mmol) and dissolve in N-N-dimethylformamide (DMF, 1 mL), then add tributylamine (8 μL) ...
Embodiment 1
[0066] Synthesis and identification of embodiment 1 tebuconazole hapten
[0067] Step 1, the synthesis of intermediate products
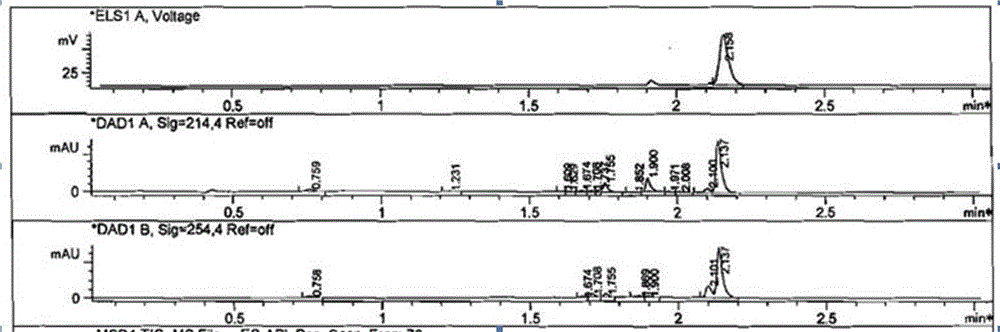
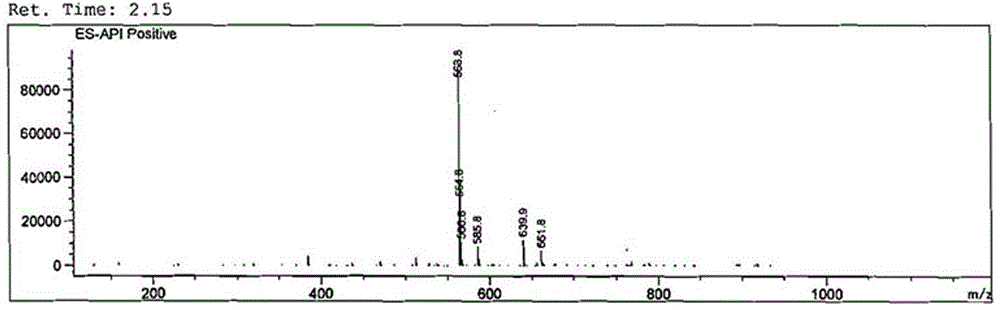
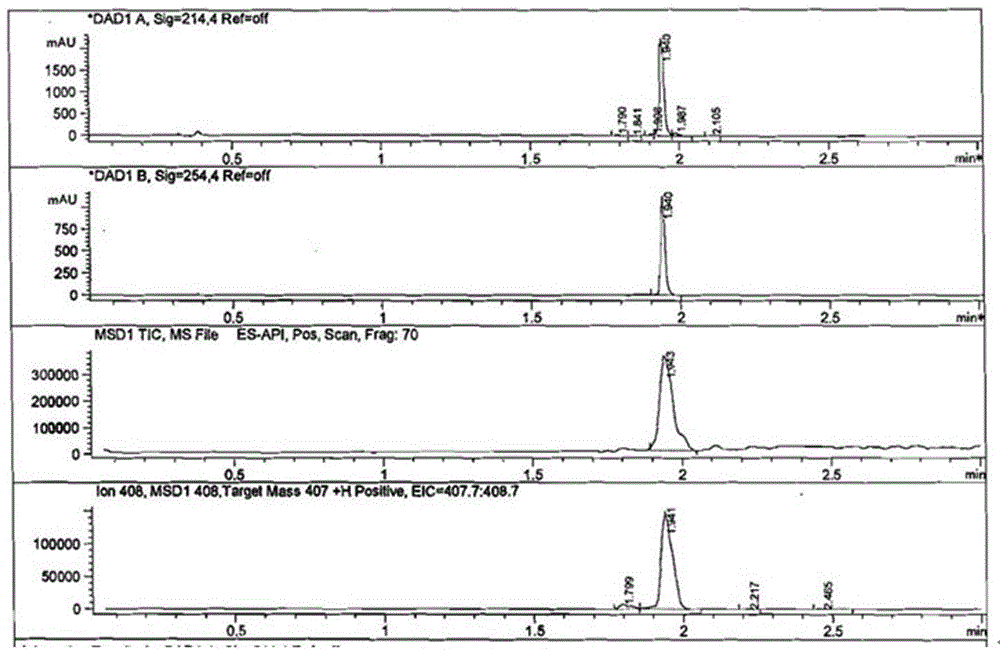
[0068] First, tebuconazole (184 mg, 0.6 mmol) was dissolved in anhydrous dichloromethane (CH 2 Cl 2 , 15 mL), then added triethylamine (C 6 h 15 N, 730 mg, 7.2 mmol), after stirring in ice bath for 5 minutes, add monoethyl succinate monoacyl chloride (C 6 h 9 ClO 3 , 988 mg, 6 mmol), stirred overnight at room temperature, washed successively with water and saturated brine (15 mL×3 respectively), dried dichloromethane with anhydrous sodium sulfate, filtered, evaporated and concentrated to dryness under reduced pressure to obtain The intermediate product (about 400mg) was directly used in the next step of hydrolysis. The structure of the product was characterized by liquid chromatography-mass spectrometry.
[0069] Step 2, the synthesis of tebuconazole hapten
[0070] The intermediate product (400 mg, 0.6 mmol) was dissolved in anhydrous meth...
Embodiment 2
[0073] Preparation and Identification of Example 2 Tebuconazole Antigen
[0074] 1. The tebuconazole hapten is coupled with hemocyanin (KLH) by the active ester method (EDC method) and used as an immunogen. The specific reaction route is as follows:
[0075]
[0076] The tebuconazole hapten is coupled with chicken ovalbumin (OVA) or bovine serum albumin (BSA) by the mixed anhydride method and used as a coating source. The specific reaction route is as follows:
[0077]
[0078] Explanation: Among them, Protein is protein.
[0079] 2. Purification of artificial antigen
[0080] The artificial antigen was desalted and purified by dialysis.
[0081] 3. Identification of artificial antigens
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com